Reduced Neurite Arborization in Primary Dopaminergic Neurons in Autism-Like Shank3B-Deficient Mice
- PMID: 39654001
- PMCID: PMC11953188
- DOI: 10.1007/s12035-024-04652-0
Reduced Neurite Arborization in Primary Dopaminergic Neurons in Autism-Like Shank3B-Deficient Mice
Abstract
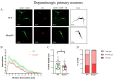
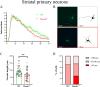
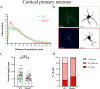
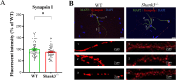
Despite many studies on dopamine changes in autism, specific alterations in midbrain dopamine neurons projecting to the striatum and cortex remain unclear. Mouse models with diverse SH3 domain and ankyrin repeat containing protein 3 (Shank3) deficiencies are used for investigating autistic symptoms and underlying neurobiological mechanisms. SHANK3 belongs to postsynaptic proteins crucial for synapse formation during development, and disruptions in SHANK3 structure could lead to impaired neurite outgrowth and altered dendritic arborization and morphology. Therefore, we aimed to investigate whether Shank3 deficiency (Shank3B) leads to changes in the morphology of primary neuronal cell cultures from dopaminergic brain regions of neonatal mouse pups and whether it results in alterations in synaptic proteins in dopaminergic nerve pathway projection areas (striatum, frontal cortex). Significantly reduced neurite outgrowth was observed in primary dopaminergic neurons from the midbrain and striatum of Shank3-deficient compared to WT mice. A decrease in Synapsin I immunofluorescence signal in the cortical neurons isolated from Shank3-deficient mice was found, although neurite arborization changes were less severe. Importantly, the deficit in the length of the longest neurite was confirmed in primary cortical neurons isolated from Shank3-deficient mice. No changes in the gene expression of synaptic proteins were observed in the striatum and frontal cortex of Shank3-deficient mice, but an altered gene expression profile of dopaminergic receptors was found. These results show structural changes of dopaminergic neurons, which may explain autistic symptomatology in the used model and provide a basis for understanding the long-term development of autistic symptoms.
Keywords: Shank3; Autism spectrum disorder; Dopaminergic neurons; Neurite outgrowth.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics Approval: All experimental procedures followed the ethical guidelines for animal experiments and were approved by the Ethical Committee of the Faculty of Medicine, Comenius University in Bratislava, Slovak Republic. The mice were handled and cared for in accordance with the Guide for the Care and Use of Laboratory Animals (N.R.C., 1996) and the European Communities Council Directive of September 22nd, 2010 (2010/63/EU, 74). Consent to Participate: Not applicable. Consent for Publication: Not applicable. Competing Interests: The authors declare no competing interests.
Figures
References
-
- Falougy HE, Filova B, Ostatnikova D, Bacova Z, Bakos J (2019) Neuronal morphology alterations in autism and possible role of oxytocin. Endocr Regul 53(1):46–54. 10.2478/enr-2019-0006 - PubMed
-
- Pavăl D (2023) The dopamine hypothesis of autism spectrum disorder: a comprehensive analysis of the evidence. Int Rev Neurobiol 173:1–42. 10.1016/bs.irn.2023.08.009 - PubMed
-
- Lu X, Song Y, Wang J, Cai Y, Peng S, Lin J, Lai B, Sun J et al (2024) Developmental dopaminergic signaling modulates neural circuit formation and contributes to autism spectrum disorder-related phenotypes. Am J Pathol S0002–9440(24)00086–5. 10.1016/j.ajpath.2024.02.014 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

