A novel single-tube LAMP-CRISPR/Cas12b method for rapid and visual detection of zoonotic Toxoplasma gondii in the environment
- PMID: 39654027
- PMCID: PMC11629535
- DOI: 10.1186/s40249-024-01266-5
A novel single-tube LAMP-CRISPR/Cas12b method for rapid and visual detection of zoonotic Toxoplasma gondii in the environment
Abstract
Background: Toxoplasma gondii oocysts, excreted in cat feces, pose a significant health risk to humans through contaminated soil and water. Rapid and accurate detection of T. gondii in environmental samples is essential for public health protection.
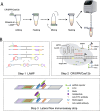
Methods: We developed a novel, single-tube detection method that integrates loop-mediated isothermal amplification (LAMP), the clustered regularly interspaced short palindromic repeats (CRISPR)/Cas12b system, and lateral flow immunoassay strips for rapid, visual identification of T. gondii. This method targets the T. gondii B1 gene, initially amplifies it with LAMP, directed by a single-guide RNA (sgRNA). It then recognizes the amplified target gene and activates trans-cleavage, cutting nearby single-stranded DNA (ssDNA) reporters. Fluorescence detection was performed using a 6-Carboxyfluorescein (FAM)-12N-Black Hole Quencher-1 (BHQ1) reporter, while Fluorescein Isothiocyanate (FITC)-12N-Biotin enabled visual detection on lateral flow strips. The method was tested for its ability to detect various T. gondii genotypes and related parasites, assessing its specificity and broad-spectrum applicability. It was further applied to real-world environmental samples to evaluate its practicality.
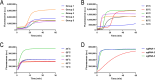
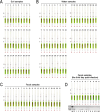
Results: The LAMP-CRISPR/Cas12b method exhibited high specificity and broad-spectrum detection capability, successfully identifying nine T. gondii genotypes and distinguishing them from 11 other parasitic species. Sensitivity testing at both molecular (plasmid) and practical (oocyst) levels showed detection limits of 10 copies/μL and 0.1 oocyst, respectively. When applied to 112 environmental samples (soil, water, and cat feces), the method demonstrated 100% sensitivity, accurately reflecting known infection rates.
Conclusions: This LAMP-CRISPR/Cas12b single-tube method offers a robust, innovative approach for monitoring zoonotic T. gondii in environmental samples, with significant implications for public health surveillance.
Keywords: Toxoplasma gondii; CRISPR/Cas12b; Environmental detection; Lateral flow immunoassay; Loop-mediated isothermal amplification; Molecular diagnostics; Zoonotic parasites.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The procedures involving animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of Shanxi Agricultural University (Approval No. SXAU-EAW-2021XM121001). Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures

References
-
- Dubey JP. Toxoplasmosis of animals and humans. 3rd ed. Boca Raton: CRC Press; 2021.
-
- Smith NC, Goulart C, Hayward JA, Kupz A, Miller CM, van Dooren GG. Control of human toxoplasmosis. Int J Parasitol. 2021;51(2–3):95–121. - PubMed
-
- Molan A, Nosaka K, Hunter M, Wang W. Global status of Toxoplasma gondii infection: systematic review and prevalence snapshots. Trop Biomed. 2019;36(4):898–925. - PubMed
MeSH terms
Supplementary concepts
Grants and funding
- 2021YFC2300800/National Key Research and Development Program of China
- 2021YFC2300802/National Key Research and Development Program of China
- 2021YFC2300804/National Key Research and Development Program of China
- LXXMsxnd202101/Shanxi Provincial Agricultural and Rural Research Program
- RFSXIHLT202101/the Research Fund of Shanxi Province for Introduced High-level Leading Talents
LinkOut - more resources
Full Text Sources
Miscellaneous

