Anaphylaxis
- PMID: 39654057
- PMCID: PMC11629490
- DOI: 10.1186/s13223-024-00926-3
Anaphylaxis
Abstract
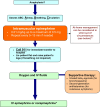
Anaphylaxis is an acute, potentially fatal systemic hypersensitivity reaction with varied mechanisms and clinical presentations. Although prompt recognition and treatment of anaphylaxis are imperative, both patients and healthcare professionals often fail to recognize and diagnose its early signs. Clinical manifestations vary widely, however, the most common signs are cutaneous symptoms, including urticaria and angioedema. Immediate intramuscular administration of epinephrine into the anterolateral thigh is first-line therapy, and is always safe even if the diagnosis is uncertain. The mainstays of long-term management include specialist assessment, allergen avoidance measures, and the provision of an epinephrine auto-injector with an individualized anaphylaxis emergency plan. This article provides an overview of the causes, clinical features, diagnosis, and acute as well as long-term management of anaphylaxis.
Keywords: Acute management; Anaphylaxis; Anaphylaxis emergency plan; Diagnosis; Epinephrine; Long-term management.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethics approval and consent to participate are not applicable to this review article. Consent for publication: Not applicable. Competing interests: Dr Elissa M. Abrams is an employee of Public Health Agency of Canada (PHAC); views expressed are her own and not those of PHAC. She is Section Head for Anaphylaxis/Food Allergy, and a Member of the Canadian Society of Allergy and Clinical Immunology (CSACI). Dr. Waleed Alqurashi has received grant funding from the Canadian Institute for Health Research. Dr. David A. Fischer is a previous President of the Canadian Society of Allergy & Clinical Immunology (CSACI). He has received consulting fees and honoraria for continuing education from AstraZeneca, GSK, Bausch Health, Valeo, AbbVie, Takeda, ALK, Pfizer, Aralez, Merck, Novartis, Sanofi, Pediapharm and Teva Pharmaceuticals. Dr. Timothy K. Vander Leek has participated in advisory boards and has received consulting fees and honoraria from Aralez/Miravo, Bausch Health, and Covis Pharma. Dr. Anne K. Ellis has participated in advisory boards for ALK-Abello, AstraZeneca, Bausch Health, LEO Pharma, Miravo, Merck, Novartis, has been a speaker for ALK-Abello, AstraZeneca, Bausch, Miravo, Medexus, Mylan, Novartis, Pfizer, Sanofi, StallergenesGreer and Regeneron. Her institution has received research grants from ALK Abello, Aralez, AstraZeneca, Bayer LLC, Medexus, Novartis, Sanofi and Regeneron. She has also served as an independent consultant to Bayer LLC, Pharming, and Regeneron. About this supplement: This article has been published as part of Allergy, Asthma & Clinical Immunology, Volume 20 Supplement 03, 2024: Practical Guide for Allergy and Immunology in Canada 2024. The full contents of the supplement are available at https://aacijournal.biomedcentral.com/articles/supplements/volume-20-supplement-3 .
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

