Cost-effectiveness of the adjuvanted RSVPreF3 vaccine among adults aged ≥60 years in the United States
- PMID: 39654072
- PMCID: PMC11633215
- DOI: 10.1080/21645515.2024.2432745
Cost-effectiveness of the adjuvanted RSVPreF3 vaccine among adults aged ≥60 years in the United States
Abstract
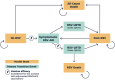
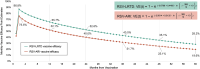
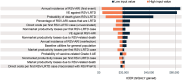
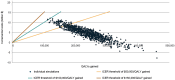
Respiratory syncytial virus (RSV) is a common cause of acute respiratory illness in individuals of all ages, with adults aged ≥60 years and adults with certain chronic conditions at increased risk of severe RSV-related outcomes. This study evaluates the cost-effectiveness of the adjuvanted RSVPreF3 vaccine versus no vaccine in adults aged ≥60 years in the United States (US). A multi-cohort Markov model was developed with a 5-year time horizon and 1-month cycle length to compare outcomes for no vaccination and one-time adjuvanted RSVPreF3 vaccination (assuming the same vaccination as for influenza vaccines). Clinical parameters (e.g., vaccine efficacy) were based on phase 3 clinical trial data over 3 seasons, with all other inputs obtained from public US sources and scientific literature. Outcomes included total and incremental quality-adjusted life year (QALY) losses and costs, as well as incremental cost-effectiveness ratios (ICERs). Sensitivity analyses were conducted to evaluate the sensitivity of results to inputs. In the base case, the model estimated that vaccinating 52.7 million adults aged ≥60 years with the adjuvanted RSVPreF3 vaccine once would result in 244,424 fewer QALY losses and an incremental societal cost of $4.5 billion over 5 years, with vaccination costs partially offset by reduced disease-related costs. From the societal perspective, adjuvanted RSVPreF3 vaccination resulted in an ICER of $18,430 per QALY gained. Results were relatively robust across sensitivity analyses and indicate that adjuvanted RSVPreF3 vaccination is a cost-effective option for the prevention of RSV in US adults aged ≥ 60 years, reducing the substantial burden within this population.
Keywords: Respiratory syncytial virus; United States; adjuvanted RSVPreF3 vaccine; cost-effectiveness; older adults; vaccination.
Conflict of interest statement
Elizabeth La, David Singer, Sara Poston, Desmond Curran, Jessica Pickett, and Frederik Verelst are employed by and hold financial equities in GSK. Daniel Molnar was employed by GSK at the time of the study. Jonathan Graham is employed by RTI Health Solutions, which received funding from GSK for the conduct of this study. All authors declare no other financial or non-financial relationships and activities.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
