A Prostate Imaging-Reporting and Data System version 2.1-based predictive model for clinically significant prostate cancer diagnosis
- PMID: 39654290
- PMCID: PMC11975180
- DOI: 10.1111/bju.16616
A Prostate Imaging-Reporting and Data System version 2.1-based predictive model for clinically significant prostate cancer diagnosis
Abstract
Objectives: To develop and validate a Prostate Imaging-Reporting and Data System (PI-RADS) version 2.1 (v2.1)-based predictive model for diagnosis of clinically significant prostate cancer (csPCa), integrating clinical and multiparametric magnetic resonance imaging (mpMRI) data, and compare its performance with existing models.
Patients and methods: We retrospectively analysed data from patients who underwent prospective mpMRI assessment using the PI-RADS v2.1 scoring system and biopsy at our institution between April 2019 and December 2023. A 'Clinical Baseline' model using patient demographics and laboratory results and an 'MRI Added' model additionally incorporating PI-RADS v2.1 scores and prostate volumes were created and validated on internal and external patients. Both models were compared against two previously published MRI-based algorithms for csPCa using area under the receiver operating characteristic curve (AUC) and decision curve analysis.
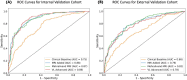
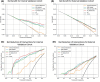
Results: A total of 1319 patients across internal and external cohorts were included. Our 'MRI Added' model demonstrated significantly improved discriminative ability (AUCinternal 0.88, AUCexternal 0.79) compared to our 'Clinical Baseline' model (AUCinternal 0.75, AUCexternal 0.68) (P < 0.001). The 'MRI Added' model also showed higher net benefits across various clinical threshold probabilities and compared to a 'biopsy all' approach, it reduced unnecessary biopsies (defined as biopsies without Gleason Grade Group ≥2 csPCa) by 27% in the internal cohort and 10% in the external cohort at a risk threshold of 25%. However, there was no significant difference in predictive ability and reduction in unnecessary biopsies between our model and comparative ones developed for PI-RADS v2 and v1.
Conclusion: Our PI-RADS v2.1-based mpMRI model significantly enhances csPCa prediction, outperforming the traditional clinical model in accuracy and reduction of unnecessary biopsies. It proves promising across diverse patient populations, establishing an updated, integrated approach for detection and management of prostate cancer.
Keywords: biopsy; magnetic resonance imaging; prostatic neoplasms; risk assessment; statistical model.
© 2024 The Author(s). BJU International published by John Wiley & Sons Ltd on behalf of BJU International. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
Figures

References
-
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin 2024; 74: 12 - PubMed
-
- Ahmed HU, El‐Shater Bosaily A, Brown LC et al. Diagnostic accuracy of multi‐parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389: 815–822 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

