Performance Evaluation of Molecular Detection of Enteroviruses: Results of 18 Years of Quality Control for Molecular Diagnostics (QCMD) External Quality Assessment Program, 2005-2022
- PMID: 39654326
- PMCID: PMC11628893
- DOI: 10.1002/jmv.70103
Performance Evaluation of Molecular Detection of Enteroviruses: Results of 18 Years of Quality Control for Molecular Diagnostics (QCMD) External Quality Assessment Program, 2005-2022
Abstract
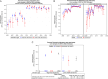
The gold standard for enterovirus (EV) detection is the polymerase chain reaction based on the detection of the 5' untranslated region of the virus. Correct detection of EV is crucial for patient and public health purposes. The performance of diagnostic and public health laboratories on molecular EV-detection was analyzed using data from the external quality assessment program distributed by Quality Control for Molecular Diagnostics (QCMD) between 2005 and 2022. Overall performance on core samples of both laboratory types has improved over the years for in-house or commercial assays (overall performance rate > 94.8%) since 2013. A similar improvement was observed for negative/specificity samples. Performance on EV-positive samples varied, with the lowest performance observed on samples containing enterovirus D68 (B3 strain) and echovirus 11. Significant differences in performance were observed between laboratory and assay types. Performance of diagnostic laboratories (91.8) was significantly higher than of public health laboratories (89.9%; p < 0.0001). Additionally, a significant higher performance of diagnostic laboratories using commercial assays was observed (92.5% vs. 91.2% for in-house assays; p < 0.0001). In contrast, the performance of public health laboratories using in-house assays (90.1%) was higher than commercial assays (89.2%; p = 0.3608). The data document the improved performance of diagnostic and public health laboratories using either commercial or in-house assays.
Keywords: QCMD; commercial assay; enterovirus; external quality assessment; in‐house assay; laboratory.
© 2024 The Author(s). Journal of Medical Virology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
A European multicentre evaluation of detection and typing methods for human enteroviruses and parechoviruses using RNA transcripts.J Med Virol. 2020 Aug;92(8):1065-1074. doi: 10.1002/jmv.25659. Epub 2020 Jan 17. J Med Virol. 2020. PMID: 31883139 Free PMC article.
-
External quality assessment for enterovirus 71 and coxsackievirus A16 detection by reverse transcription-PCR using armored RNA as a virus surrogate.J Clin Microbiol. 2011 Oct;49(10):3591-5. doi: 10.1128/JCM.00686-11. Epub 2011 Aug 24. J Clin Microbiol. 2011. PMID: 21865426 Free PMC article.
-
Epidemic 2014 enterovirus D68 cross-reacts with human rhinovirus on a respiratory molecular diagnostic platform.PLoS One. 2015 Mar 23;10(3):e0118529. doi: 10.1371/journal.pone.0118529. eCollection 2015. PLoS One. 2015. PMID: 25799541 Free PMC article.
-
External Quality Assessment (EQA) for Molecular Diagnostics of Zika Virus: Experiences from an International EQA Programme, 2016⁻2018.Viruses. 2018 Sep 13;10(9):491. doi: 10.3390/v10090491. Viruses. 2018. PMID: 30216988 Free PMC article. Review.
-
CM-Path Molecular Diagnostics Forum-consensus statement on the development and implementation of molecular diagnostic tests in the United Kingdom.Br J Cancer. 2019 Oct;121(9):738-743. doi: 10.1038/s41416-019-0588-1. Epub 2019 Oct 2. Br J Cancer. 2019. PMID: 31575975 Free PMC article. Review.
References
-
- Picornavirus Study Group , accessed June 2024, 2024, http://www.picornastudygroup.com/.
-
- Harvala H., Broberg E., Benschop K., et al., “Recommendations for Enterovirus Diagnostics and Characterisation Within and Beyond Europe,” Journal of Clinical Virology 101 (2018): 11–17. - PubMed
-
- Harvala H., Jasir A., Penttinen P., Pastore Celentano L., Greco D., and Broberg E., “Surveillance and Laboratory Detection for Non‐Polio Enteroviruses in the European Union/European Economic Area, 2016,” Eurosurveillance 22, no. 45 (2017): 16–00807, 10.2807/1560-7917.ES.2017.22.45.16-00807. - DOI - PMC - PubMed
-
- Bubba L., Broberg E. K., Jasir A., et al., “Circulation of Non‐Polio Enteroviruses in 24 EU and EEA Countries Between 2015 and 2017: A Retrospective Surveillance Study,” Lancet Infectious Diseases 20, no. 3 (2020): 350–361. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

