The Impact of Early Diagenesis on Biosignature Preservation in Sulfate Evaporites: Insights From Messinian (Late Miocene) Gypsum
- PMID: 39654375
- PMCID: PMC11629073
- DOI: 10.1111/gbi.70007
The Impact of Early Diagenesis on Biosignature Preservation in Sulfate Evaporites: Insights From Messinian (Late Miocene) Gypsum
Abstract
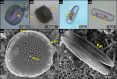
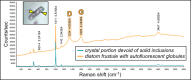
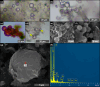
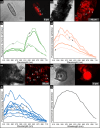
Due to their fast precipitation rate, sulfate evaporites represent excellent repositories of past life on Earth and potentially on other solid planets. Nevertheless, the preservation potential of biogenic remains can be compromised by extremely fast early diagenetic processes. The upper Miocene, gypsum-bearing sedimentary successions of the Mediterranean region, that formed ca. 6 million years ago during the Messinian salinity crisis, represent an excellent case study for investigating these diagenetic processes at the expense of organic matter and associated biominerals. Several gypsum crystals from the Northern Mediterranean were studied by means of destructive and non-destructive techniques in order to characterize their solid inclusion content and preservation state. In the same crystal, excellently preserved microfossils coexist with strongly altered biogenic remains. Altered remains are associated with authigenic minerals, especially clays. The results demonstrate that a significant fraction of organic matter and associated biominerals (notably biogenic silica) underwent early diagenetic modification. The latter was likely triggered by bottom sulfidic conditions when the growth of gypsum was interrupted. These results have significant implications for the interpretation of the Messinian Salt Giant.
Keywords: Mars; Messinian; authigenic clays; biosignatures; salt giants; sulfate evaporites.
© 2024 The Author(s). Geobiology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









Similar articles
-
Morphological biosignatures in gypsum: diverse formation processes of Messinian (∼6.0 Ma) gypsum stromatolites.Astrobiology. 2013 Sep;13(9):870-86. doi: 10.1089/ast.2013.1021. Astrobiology. 2013. PMID: 24047112
-
Biosignature Preservation Potential in Playa Evaporites: Impacts of Diagenesis and Implications for Mars Exploration.Astrobiology. 2018 Nov;18(11):1460-1478. doi: 10.1089/ast.2018.1849. Epub 2018 Aug 20. Astrobiology. 2018. PMID: 30124326
-
Planning Implications Related to Sterilization-Sensitive Science Investigations Associated with Mars Sample Return (MSR).Astrobiology. 2022 Jun;22(S1):S112-S164. doi: 10.1089/AST.2021.0113. Epub 2022 May 19. Astrobiology. 2022. PMID: 34904892
-
The Desiccation and Catastrophic Refilling of the Mediterranean: 50 Years of Facts, Hypotheses, and Myths Around the Messinian Salinity Crisis.Ann Rev Mar Sci. 2025 Jan;17(1):485-509. doi: 10.1146/annurev-marine-021723-110155. Epub 2024 Nov 25. Ann Rev Mar Sci. 2025. PMID: 39106583 Review.
-
Preservation of martian organic and environmental records: final report of the Mars biosignature working group.Astrobiology. 2011 Mar;11(2):157-81. doi: 10.1089/ast.2010.0506. Epub 2011 Mar 9. Astrobiology. 2011. PMID: 21417945 Review.
References
-
- Agustí, S. , Krause J. W., Marquez I. A., Wassmann P., Kristiansen S., and Duarte C. M.. 2018. “Arctic (Svalbard Islands) Active and Exported Diatom Stocks and Cell Health Status.” Biogeosciences Discussions 17: 35–45. 10.5194/bg-2018-459. - DOI
-
- Aloisi, G. , Guibourdenche L., Natalicchio M., et al. 2022. “The Geochemical Riddle of ‘Low‐Salinity Gypsum’ Deposits.” Geochimica et Cosmochimica Acta 327: 247–275. 10.1016/j.gca.2022.03.033. - DOI
-
- Badaut, D. , and Risacher F.. 1983. “Authigenic Smectite on Diatom Frustules in Bolivian Saline Lakes.” Geochimica et Cosmochimica Acta 47: 363–375. 10.1016/0016-7037(83)90259-4. - DOI
-
- Bae, H. , Park J., Ahn H., and Khim J. S.. 2020. “Shift in Benthic Diatom Community Structure and Salinity Thresholds in a Hypersaline Environment of Solar Saltern, Korea.” Algae 35, no. 4: 361–373. 10.4490/algae.2020.35.12.4. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

