Geraniol Ameliorates Pentylenetetrazol-Induced Epilepsy, Neuroinflammation, and Oxidative Stress via Modulating the GABAergic Tract: In vitro and in vivo studies
- PMID: 39654600
- PMCID: PMC11627104
- DOI: 10.2147/DDDT.S481985
Geraniol Ameliorates Pentylenetetrazol-Induced Epilepsy, Neuroinflammation, and Oxidative Stress via Modulating the GABAergic Tract: In vitro and in vivo studies
Abstract
Introduction: Geraniol (Ger), a monoterpene, is a common constituent of several essential oils. This study explored the anticonvulsant effect of Ger in-vitro using nerve growth factor (NGF) prompted PC12 cell injured by Glutamate (Glu) and in-vivo using Pentylenetetrazole (PTZ)-induced kindling through the GABAergic pathway.
Materials: To assess the effect of Ger on NGF prompted PC12 cells injured by Glu, Ger at concentrations of 25, 50, 100, 200 and 400 μg/mL was used. GABA, 5-HT, IL-1β, IL-4, and TNF-α levels and the gene expressions of GABAA-Rα1, NMDAR1, GAD 65, GAD 67, GAT 1 and GAT 3 were measured in NGF-induced PC12 cells treated with Ger (100, and 200 μg/mL). Mice were randomly separated into five groups. Normal and PTZ groups in which mice were injected with saline or PTZ, respectively. PTZ + Ger 100, PTZ + Ger 200 and PTZ + SV groups in which mice orally administered Ger or sodium valproate (SV), respectively, then injected with PTZ.
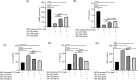
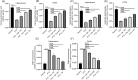
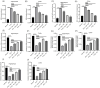
Results: Ger up to 400 μg/mL did not display any toxicity or injury in PC12 cells. Ger (100 to 200 μg/mL) reduced the injury induced by Glu, increased the gene expression of GABAA-Rα1, GAD65 and GAD67 and decreased GAT 1, GAT 3 and NMDAR1 expression in NGF-induced PC12 cells damaged by Glu. Ger (100 to 200 μg/mL) increased GABA and reduced TNF-α, IL-4 and IL-1β levels in NGF-induced PC12 cells injured by Glu. As for the in-vivo results, Ger increased GABA, GAD, GAT 1 and 3 and lowered GABA T. Ger mitigated MDA, NO, IL-1β, IL-6, TNF-α and IFN-γ, GFAP, caspase-3, and -9 levels and Bax gene expression and escalated GSH, SOD, catalase, BDNF and Bcl2 gene expression.
Conclusion: Ger reduced the oxidative stress status, neuroinflammation and apoptosis and activated GABAergic neurotransmission, which might clarify its anticonvulsant. Ger protects animals against PTZ prompted kindling as established by the enhancement in short term as well as long-term memory. Ger mitigated the injury induced by Glu in NGF prompted PC12 cell.
Keywords: GABAergic; apoptosis; geraniol; neuroinflammation; oxidative stress; pentylenetetrazole.
© 2024 Younis et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Chen W, Viljoen AM. Geraniol — a review of a commercially important fragrance material. S Afr J Bot. 2010;76(4):643–651. doi: 10.1016/j.sajb.2010.05.008 - DOI
-
- Lira MH, Andrade Júnior FP, Moraes GF, Macena G, Pereira F, Lima IO. Antimicrobial activity of geraniol: an integrative review. J Essent Oil Res. 2020;32(3):187–197. doi: 10.1080/10412905.2020.1745697 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

