Polymicrobial interactions influence Mycobacterium abscessus co-existence and biofilm forming capabilities
- PMID: 39654682
- PMCID: PMC11627178
- DOI: 10.3389/fmicb.2024.1484510
Polymicrobial interactions influence Mycobacterium abscessus co-existence and biofilm forming capabilities
Abstract
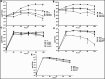
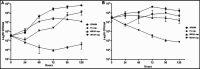
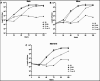
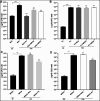
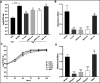
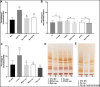
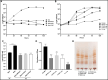
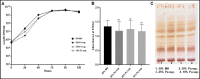
The lungs of patients with cystic fibrosis (CF) are vulnerable to persistent polymicrobial colonization by bacterial pathogens including Pseudomonas aeruginosa, Staphylococcus aureus, and the non-tuberculous mycobacterium (NTM) Mycobacterium abscessus. The polymicrobial milieu within the CF lung impacts individual species fitness, influences biofilm-forming capabilities, pathogenicity, production of virulence factors and even antimicrobial responses, all potentially compromising therapeutic success. Interaction studies among these CF pathogens are very limited, especially studies on the influences of P. aeruginosa and S. aureus on M. abscessus co-existence and virulence. Based on the little known thus far about coinfection of these pathogens, we hypothesize that the co-existence of P. aeruginosa and S. aureus alters M. abscessus virulence and phenotypic characteristics. We evaluated the direct (co-culture) and indirect (using supernatant) effects of P. aeruginosa and S. aureus on M. abscessus growth rate, biofilm formation, macrophage internalization and glycopeptidolipids (GPL) expression. Our observations indicate that P. aeruginosa and S. aureus exert a competitive behavior toward M. abscessus during direct contact or indirect interaction in-vitro, probably as is the case of polymicrobial infections in the lungs of patients with CF. This is the first report that demonstrates S. aureus inhibitory effects on M. abscessus growth and biofilm forming capabilities. Collectively, co-culture studies enhance our understanding of polymicrobial interactions during coinfection and can guide to establish better management of coinfections and treatment strategies for M. abscessus.
Keywords: Mycobacterium abscessus; Pseudomonas aeruginosa; Staphylococcus aureus; biofilm formation; co-culture; coinfection; polymicrobial interaction.
Copyright © 2024 Nandanwar, Gu, Gibson and Neely.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

