Consensus Recommendations for the Reconstitution and Aesthetic Use of Poly-D,L-Lactic Acid Microspheres
- PMID: 39654687
- PMCID: PMC11626391
- DOI: 10.2147/CCID.S497691
Consensus Recommendations for the Reconstitution and Aesthetic Use of Poly-D,L-Lactic Acid Microspheres
Abstract
Poly-D,L-lactic acid (PDLLA) microspheres, marketed globally as Aesthefill® (Regen Biotech, Seoul, South Korea), are recognized for their biocompatible and biostimulatory properties, positioning them as a preferred option in aesthetic medicine. This article presents consensus recommendations from Brazilian experts on the reconstitution and clinical application of PDLLA for facial and non-facial treatments. Developed using a modified Delphi method with contributions from leading dermatologists and plastic surgeons, the consensus outlines protocols for reconstitution, injection techniques, and patient management. Key recommendations include reconstitution with 7-8 mL of sterile water for injection, the addition of lidocaine to improve patient comfort, and a preference for targeting the superficial subcutaneous layer. Dosing guidelines are specifically tailored to each treatment area and the desired degree of correction, underscoring the importance of personalized treatment plans. Maintenance treatments are advised at biennial intervals or at shorter intervals for patients exhibiting accelerated collagen degradation. The consensus also highlights the need for proper training and patient screening to minimize adverse effects, such as nodules and granulomas. This comprehensive guide aims to standardize the use of PDLLA, prioritizing patient safety and optimizing outcomes. While clinical trials evaluating PDLLA's aesthetic indications remain limited, these evidence-based guidelines bridge the gap by offering practical protocols grounded in clinical expertise. Further research is encouraged to validate these recommendations and explore new applications for PDLLA in aesthetic medicine.
Keywords: collagen stimulator; consensus; expert recommendations; filler; poly-D,L-lactic acid; polylactic acid.
Plain language summary
Poly-D, L-lactic acid (PDLLA) microspheres are small particles used in aesthetic medicine to improve skin appearance by stimulating collagen production. This summary outlines expert recommendations from experts on preparing and using PDLLA for facial and body treatments. Why was the study done? The study aimed to create standardized guidelines to help practitioners achieve the best results and ensure patient safety when using PDLLA for cosmetic purposes. What did the researchers do and find? They used a method called the Delphi approach to gather and refine recommendations on the reconstitution and injection of PDLLA. By consensus, the experts who participated in this study recommend using of sterile water for injection to prepare the PDLLA and adding lidocaine to reduce discomfort during injections. Specific techniques, such as linear retrograde and fanning methods, are advised for different areas of the face and body. The experts have also provided dosing guidelines and emphasize the need for individualized treatment plans. What do these results mean? The recommendations help practitioners use PDLLA more effectively and safely, providing better aesthetic outcomes for patients. Regular maintenance treatments are suggested every two years, with more frequent sessions for those with faster collagen loss. Proper training and careful patient screening are essential to avoid complications such as nodules or granulomas.
© 2024 Magacho-Vieira et al.
Conflict of interest statement
Dr F.N. Magacho-Vieira is a medical director for Derma Dream Corporation Brazil and was a regular speaker for Galderma, until May, 2024. A. Soares Jr, H.C.L. Alvarenga, I.R.A. Oliveira Jr. J.A.C. Daher, J.V.M.P. Napoli, J.P.A. Serra, and S.C. Provázio are speakers for Derma Dream Corporation Brazil. A. Soares Jr is also a speaker for Medsystem Brazil and Evopharma Brazil. Abrahao Vieira reports being a Speaker for Dermadream Corporation Brazil. João Napoli reports non-financial support from Derma Dream Corporation Brazil, during the conduct of the study; non-financial support from Dream Corporation Brazil, outside the submitted work. The authors report no other conflicts of interest in this work.
Figures


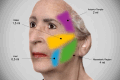
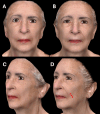
References
-
- Azarkevich PN, Gorskaya AA. Physicochemical Characteristics and Hydrolytic Degradation of Polylactic Acid Dermal Fillers: a Comparative Study. Cosmetics. 2023;10(4):110. doi: 10.3390/cosmetics10040110 - DOI
-
- Lin CY, Lin JY, Yang DY, Lee SH, Kim JY, Kang M. Efficacy and safety of poly-D,L-lactic acid microspheres as subdermal fillers in animals. Plast Aesthet Res. 2019;6:16. doi: 10.20517/2347-9264.2019.23 - DOI
-
- Doe J, Smith A. Compositions of Injectable Poly-D,L-lactic Acid and Injectable Poly-L-lactic Acid. Aesthetic Surg J. 2024;44(3):199–207. doi: 10.1093/asj/sjab023 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

