Cannabis combined with oxycodone for pain relief in fibromyalgia pain: a randomized clinical self-titration trial with focus on adverse events
- PMID: 39654798
- PMCID: PMC11625723
- DOI: 10.3389/fpain.2024.1497111
Cannabis combined with oxycodone for pain relief in fibromyalgia pain: a randomized clinical self-titration trial with focus on adverse events
Abstract
Objectives: We determined whether adding cannabis to oxycodone for chronic non-cancer pain management could reduce treatment-related adverse effects (AEs) while maintaining effective analgesia.
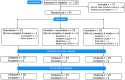
Methods: In this open-label study, fibromyalgia patients aged ≥18 years were randomized to receive 5 mg oxycodone tablets (max. four times/day), 150 mg of inhaled cannabis containing 6.3% Δ9-tetrahydrocannabinol and 8% cannabidiol (max. times inhalation sessions/day), or a combination of both for 6 weeks. The primary endpoint was treatment-related adverse events, assessed using a 10-point composite adverse event (cAE) score; additionally, we recorded daily reported pain relief and daily tablet and cannabis consumption.
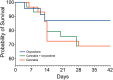
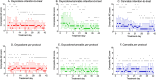
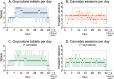
Results: In total, 23 patients were treated with oxycodone, 29 with cannabis, and 29 with the oxycodone/cannabis combination. Three patients from the oxycodone group (13%) and 18 patients from the cannabis groups (31%, 9 in each group) withdrew from the trial within 2-3 weeks because of the severity of AEs. There were no differences in treatment-related cAE scores among the three groups that completed the study (p = 0.70). The analgesic responder rate showed a ≥1- point reduction in pain in 50% and a ≥2-point reduction in 20% of patients, while 50% of patients experienced no treatment benefit. The combination treatment reduced oxycodone tablet consumption by 35% (p = 0.02), but it did not affect the number of cannabis inhalation sessions.
Conclusions: Cannabis combined with oxycodone offered no advantage over either treatment alone, except for a reduction in opioid tablet intake; however, the overall drug load was the highest in the combination group. Moreover, cannabis was poorly tolerated and led to treatment discontinuation in one-third of participants treated with cannabis.
Clinical trial registration: The trial was registered at the WHO International Clinical Trials Registry Platform (trialsearch.who.int) on July 26, 2019, identifier NL7902.
Keywords: adverse (side) effects; analgesia; cannabis; oxycodone; self-titration.
© 2024 van Dam, Kramers, Schellekens, Bouvy, van Dorp, Kowal, Olofsen, Dahan, Niesters and van Velzen.
Conflict of interest statement
AD reported receiving personal fees from Trevena and Enalare outside the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
-
- Dahan A, Niesters M, Smith T, Overdyk F. “Opioids”. In: Barash, Cullen and Stoelting's Clinical Anesthesia. 9th ed. Philadelphia, PA: Wolters Kluwer; (2023). p. 505–26.
LinkOut - more resources
Full Text Sources

