A Novel Triptolide Nano-Liposome with Mitochondrial Targeting for Treatment of Hepatocellular Carcinoma
- PMID: 39654802
- PMCID: PMC11626209
- DOI: 10.2147/IJN.S498099
A Novel Triptolide Nano-Liposome with Mitochondrial Targeting for Treatment of Hepatocellular Carcinoma
Abstract
Background: Modern pharmacological studies have demonstrated that although triptolide (TP) is effective against hepatocellular carcinoma, it has poor water solubility and more toxic side effects. In this study, we used triptolide (TP), a bioactive constituent in Tripterygium wilfordii Hook F, as a model drug to develop a novel nano-liposome drug delivery system for the treatment of liver tumours.
Methods: We constructed a functionally-modified triptolide liposome (FA+TPP-TP-Lips) using the film-dispersion method and investigated its physicochemical properties, mitochondrial targeting of hepatic tumour cells, in vitro and in vivo anti-hepatic tumour activity and its mechanism.
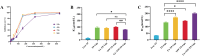
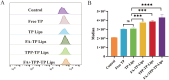
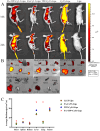
Results: The prepared FA+TPP-TP-Lips had a particle size of 99.28 ± 5.7 nm, a PDI of 0.20 ± 0.02, a zeta potential of 1.2 ± 0.08 mV, and an encapsulation rate of 74.37% ± 1.07%.FA+TPP-TP-Lips facilitates the cellular uptake of drug delivery systems and improves their targeted delivery to mitochondria. The results of cell efficacy showed that FA+TPP-TP-Lips significantly inhibited the growth of liver cancer cells, decreased mitochondrial membrane potential, and increased intracellular ROS, thus enhancing the highest apoptosis rate of liver cancer cells. The targeted liposomes (FA-TP-Lips, TPP-TP-Lips, and FA+TPP-TP-Lips) had some degree of inhibitory migration effect on Huh-7 cells relative to the unmodified TP-Lips. Studies on tumor-bearing mice demonstrated that FA+ TPP-TP-Lips effectively accumulated in tumor tissues and significantly inhibited the growth of subcutaneous tumors, achieving a tumor inhibition rate of 79.37%. FA+ TPP-TP-Lips demonstrated an enhanced anti-liver tumor effect and significantly mitigated the hepatotoxicity and systemic toxicity associated with TP.
Conclusion: In summary, the results of this study can provide a feasible solution for improving the mitochondrial targeting of nano-liposomes, and lay a foundation for further developing a novel nano targeting preparation of triptolide for the treatment of hepatocellular carcinoma.
Keywords: Folic acid; Hepatocellular Carcinoma; Mitochondrial Targeting; Triphenylphosphine ion; Triptolide.
© 2024 Zhou et al.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures










References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

