Induction of Innate Immune Memory in LPS-Primed Microglial Cells by Water-Soluble Chitosan
- PMID: 39654846
- PMCID: PMC11628173
- DOI: 10.1155/bmri/8027006
Induction of Innate Immune Memory in LPS-Primed Microglial Cells by Water-Soluble Chitosan
Abstract
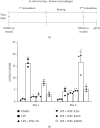
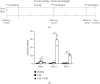
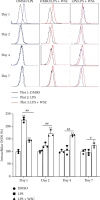
Innate immune memory or trained immunity refers to a long-lasting response of the innate immune cells against repeated exposure to the homogenous or heterogenous infectious agent. The trained immunity is induced through epigenetic modification and is characterized by the change of both intracellular immunological signaling and cellular metabolism. Recently, different groups have tried to establish protocols to generate trained innate immune cells. However, the molecular basis of innate memory induction remains poorly understood. Here, we evaluated the impact of water-soluble chitosan on the innate immune memory induction in microglial cells primed with LPS. The trained-immune response was accessed by measuring proinflammatory markers, metabolic change, and epigenetic modification. We showed that the stimulation/restimulation with LPS only caused a robust reduction of iNOS, and proinflammatory cytokines, indicating induced immune tolerance. In contrast, the treatment of chitosan induces long-lasting memory microglial cells accompanied by a high level of iNOS, increased lactate production, induced epigenetic modification, and the upregulation of proinflammatory cytokines upon further exposure to the same stimulus. These findings suggest that chitosan induces microglial-trained immunity by targeting distinct epigenetic and metabolic pathways; therefore, chitosan treatment may provide a novel approach for targeting innate immunity towards a memory-like response in an in vitro model.
Keywords: microglia; trained immunity; water-soluble chitosan.
Copyright © 2024 Vo Thuy Anh Thu et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

