Immunophenotyping characteristics and clinical outcome of COVID-19 patients treated with azvudine during the Omicron surge
- PMID: 39654887
- PMCID: PMC11625784
- DOI: 10.3389/fimmu.2024.1465238
Immunophenotyping characteristics and clinical outcome of COVID-19 patients treated with azvudine during the Omicron surge
Abstract
Background: Little is known about immunophenotyping characteristics and clinical outcomes of COVID-19 patients treated with azvudine during the Omicron variant surge.
Methods: This study enrolled patients diagnosed with COVID-19 from December 2022 to February 2023. The primary outcome was defined as all-cause mortality, along with a composite outcome reflecting disease progression. The enrolled patients were followed for a period of 60 days from their admission.
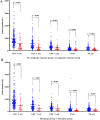
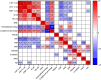
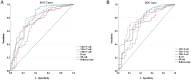
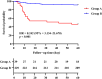
Results: A total of 268 COVID-19 patients treated with azvudine were enrolled in this retrospective study. The study found that the counts of lymphocyte subsets were significantly reduced in the composite outcome and all-cause mortality groups compared to the non-composite outcome and discharge groups (all p < 0.001). Correlation analysis revealed a negative association between lymphocyte subsets cell counts and inflammatory markers levels. The receiver operating characteristic (ROC) curve analysis identified low CD4+ T cell count as the most significant predictor of disease progression and all-cause mortality among the various lymphocyte subsets. Additionally, both the Kaplan-Meier curve and multivariate regression analysis demonstrated that low CD4+ T cell count level (< 156.00 cells/μl) was closely associated with all-cause mortality in COVID-19 patients treated with azvudine.
Conclusions: A low CD4+ T cell count may serve as a significant predictive indicator for identifying COVID-19 patients receiving azvudine treatment who are at an elevated risk of experiencing adverse outcomes. These findings may offer valuable insights for physicians in optimizing the administration of azvudine.
Keywords: CD4+ T cell; COVID-19; azvudine; lymphocyte subsets; mortality.
Copyright © 2024 Qiu, Song, Zhang, Zou, Pang and Nian.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Modes ME, Directo MP, Melgar M, Johnson LR, Yang H, Chaudhary P, et al. . Clinical characteristics and outcomes among adults hospitalized with laboratory-confirmed SARS-CoV-2 infection during periods of B.1.617.2 (Delta) and B.1.1.529 (Omicron) variant predominance-one hospital, California, July 15-September 23, 2021, and December 21, 2021-January 27, 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:217–23. doi: 10.15585/mmwr.mm7106e2 - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials

