Cost-effectiveness analysis of benmelstobart, anlotinib, and chemotherapy in extensive-stage small-cell lung cancer
- PMID: 39654891
- PMCID: PMC11625741
- DOI: 10.3389/fimmu.2024.1477146
Cost-effectiveness analysis of benmelstobart, anlotinib, and chemotherapy in extensive-stage small-cell lung cancer
Abstract
Background: The ETER701 trial assessed the efficacy and safety of benmelstobart combined with anlotinib plus etoposide/cisplatin (BEN-AL-EC) as a first-line therapy for extensive-stage small-cell lung cancer (ES-SCLC). Results indicated that BEN-AL-EC, when compared with placebo in combination with etoposide/cisplatin (PLB-EC), significantly enhanced both progression-free and overall survival rates, while demonstrating an acceptable safety profile among patients with ES-SCLC. However, BEN-AL-EC is expensive, necessitating its cost-effectiveness analysis.
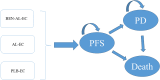
Methods: A Markov model with three health states was developed to evaluate the cost-effectiveness of BEN-AL-EC, AL-EC and PLB-EC for the treatment of ES-SCLC from the perspective of the Chinese healthcare system. Drug costs were derived from national tender prices, whereas other costs and utility values were derived from published literature. The key outcomes assessed included total costs, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs). Sensitivity analyses, including one-way and probabilistic analyses, were performed to assess the robustness of the model.
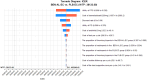
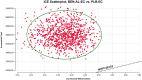
Results: The total cost of BEN-AL-EC was $55,117.42, yielding 1.09 QALYs, whereas that of PLB-EC was $15,238.15, yielding 0.71 QALYs. The ICER of BEN-AL-EC compared with PLB-EC was $106,249.42 per QALY gained. At a willingness-to-pay threshold of $38,133 per QALY, BEN-AL-EC had a 0% probability of being cost-effective relative to PLB-EC. The key parameters influencing these outcomes included utility values for PFS, the cost of benmelstobart, and the discount rate.
Conclusion: From the perspective of the Chinese healthcare system, BEN-AL-EC as a first-line treatment for ES-SCLC is unlikely to be cost-effective when compared with PLB-EC.
Keywords: anlotinib; benmelstobart; chemotherapy; cost-effectiveness; extensive-stage small-cell lung cancer; first-line treatment.
Copyright © 2024 You, Luo, Lu, Chen and He.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Cost-effectiveness of benmelstobart and anlotinib plus chemotherapy as first-line therapy for extensive-stage small cell lung cancer in China.Sci Rep. 2025 Mar 24;15(1):10147. doi: 10.1038/s41598-025-91540-9. Sci Rep. 2025. PMID: 40128536 Free PMC article.
-
Cost-effectiveness of benmelstobart-anlotinib-chemotherapy in extensive-stage small-cell lung cancer: A comparative analysis across United States and Chinese healthcare systems.Int J Clin Pharm. 2025 Jul 24. doi: 10.1007/s11096-025-01968-2. Online ahead of print. Int J Clin Pharm. 2025. PMID: 40705178
-
Benmelstobart, anlotinib and chemotherapy in extensive-stage small-cell lung cancer: a randomized phase 3 trial.Nat Med. 2024 Oct;30(10):2967-2976. doi: 10.1038/s41591-024-03132-1. Epub 2024 Jul 11. Nat Med. 2024. PMID: 38992123 Free PMC article. Clinical Trial.
-
Cost-effectiveness of the combination of immunotherapy and chemotherapy for extensive-stage small-cell lung cancer: a systematic review.BMC Health Serv Res. 2023 Jun 26;23(1):691. doi: 10.1186/s12913-023-09727-7. BMC Health Serv Res. 2023. PMID: 37365540 Free PMC article.
-
Anlotinib combined with tislelizumab in the treatment of primary small cell neuroendocrine carcinoma of the prostate: a case report and literature review.Front Immunol. 2024 Dec 23;15:1510069. doi: 10.3389/fimmu.2024.1510069. eCollection 2024. Front Immunol. 2024. PMID: 39763647 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

