Cilostazol geno-protective effects mitigate carbamazepine-induced genotoxicity in human cultured blood lymphocytes
- PMID: 39654995
- PMCID: PMC11626827
- DOI: 10.1016/j.toxrep.2024.101814
Cilostazol geno-protective effects mitigate carbamazepine-induced genotoxicity in human cultured blood lymphocytes
Abstract
Background: Carbamazepine is one of the most widely used antiepileptic drugs. Carbamazepine has been shown to be toxic to cells. Cilostazol, an antiplatelet agent, has known antioxidant, antiproliferative, anti-inflammatory, and anti-tumor effects.
Objective: This study aimed to explore whether carbamazepine and cilostazol exert genotoxic and/or cytotoxic effects in human cultured blood lymphocytes and the impact of combining both drugs on such effects.
Methods: Genotoxicity was examined using sister chromatid exchange (SCE) assay, while cytotoxicity was evaluated by cell kinetic assays (mitotic and proliferative indices).
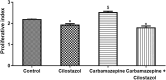
Results: Study findings have revealed that carbamazepine markedly increased SCEs (p<0.01), while cilostazol significantly decreased their frequencies (p<0.01). In addition, the frequency of SCEs of the combination of both drugs was similar to that of the control group (p>0.05). Carbamazepine increased the cell proliferative index (p<0.01) while cilostazol decreased it (p<0.01). The proliferative index was normalized to the control level when both drugs were combined.
Conclusion: We suggest that cilostazol has the potential to protect human lymphocytes from carbamazepine-induced toxic effects.
Keywords: Carbamazepine; Cell kinetic assays; Cilostazol; Cytotoxic; Genotoxic; Sister chromatid exchange.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- C.S. Jasdave Maan, T.H. vi Duong, A. Saadabadi, Continuing Education Activity, n.d. 〈https://www.ncbi.nlm.nih.gov/books/NBK482455/〉.
-
- García-Medina S., Galar-Martínez M., Gómez-Oliván L.M., Torres-Bezaury R.M. del C., Islas-Flores H., Gasca-Pérez E. The relationship between cyto-genotoxic damage and oxidative stress produced by emerging pollutants on a bioindicator organism (Allium cepa): The carbamazepine case. Chemosphere. 2020;253 doi: 10.1016/j.chemosphere.2020.126675. - DOI - PubMed
LinkOut - more resources
Full Text Sources

