Creating a plasma coordination center to support COVID-19 outpatient trials across a national network of hospital blood banks
- PMID: 39655012
- PMCID: PMC11626586
- DOI: 10.1017/cts.2024.642
Creating a plasma coordination center to support COVID-19 outpatient trials across a national network of hospital blood banks
Abstract
Introduction: In response to the COVID-19 pandemic, we rapidly implemented a plasma coordination center, within two months, to support transfusion for two outpatient randomized controlled trials. The center design was based on an investigational drug services model and a Food and Drug Administration-compliant database to manage blood product inventory and trial safety.
Methods: A core investigational team adapted a cloud-based platform to randomize patient assignments and track inventory distribution of control plasma and high-titer COVID-19 convalescent plasma of different blood groups from 29 donor collection centers directly to blood banks serving 26 transfusion sites.
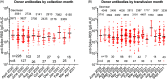
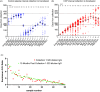
Results: We performed 1,351 transfusions in 16 months. The transparency of the digital inventory at each site was critical to facilitate qualification, randomization, and overnight shipments of blood group-compatible plasma for transfusions into trial participants. While inventory challenges were heightened with COVID-19 convalescent plasma, the cloud-based system, and the flexible approach of the plasma coordination center staff across the blood bank network enabled decentralized procurement and distribution of investigational products to maintain inventory thresholds and overcome local supply chain restraints at the sites.
Conclusion: The rapid creation of a plasma coordination center for outpatient transfusions is infrequent in the academic setting. Distributing more than 3,100 plasma units to blood banks charged with managing investigational inventory across the U.S. in a decentralized manner posed operational and regulatory challenges while providing opportunities for the plasma coordination center to contribute to research of global importance. This program can serve as a template in subsequent public health emergencies.
Keywords: COVID-19; clinical trial management; cloud-based platform; convalescent plasma; decentralized clinical trial; investigational drug services; supply chain management.
© The Author(s) 2024.
Conflict of interest statement
TJG is a paid consultant and employee of Fenwal, a Fresenius Kabi company (2021–2023); Employee of Werfen (2023-present). AC is on a Scientific Advisory Board of Sabtherapeutics (cow-derived human immunoglobulins COVID-19 treatment and other infectious diseases) and Ortho Diagnostics Speakers Bureau. MAH contracts from Gilead Sciences, Insmed, AN2 Therapeutics, AstraZeneca to the University of Cincinnati, outside the submitted work. EB is a member of the FDA Blood Products Advisory Committee. SS reports research grants; F2G, Cidara, Ansun, Zeteo: personal fees as consultant, advisory board, data safety monitoring board member; Celltrion, Adagio, Immunome, Karius, Pfizer, Scynexis, Adamis, Karyopharm, Intermountain Health: Stock options: Immunome. All other authors report no relevant disclosures.
Figures



References
-
- Administration USFaD. In: Administration USFaD ed.: USFDA; 2003. https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ap....
LinkOut - more resources
Full Text Sources
