Hepatocellular carcinoma risk scores for non-viral liver disease: A systematic review and meta-analysis
- PMID: 39655093
- PMCID: PMC11625341
- DOI: 10.1016/j.jhepr.2024.101227
Hepatocellular carcinoma risk scores for non-viral liver disease: A systematic review and meta-analysis
Abstract
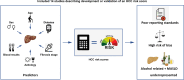
Background & aims: Hepatocellular carcinoma (HCC) risk prediction models may provide a more personalised approach to surveillance for HCC among patients with cirrhosis. This systematic review aims to summarise the performance of HCC prediction models in patients with non-viral chronic liver disease.
Method: The study was prospectively registered with PROSPERO (ID: CRD42022370078) and reported in accordance with PRISMA guidelines. MEDLINE and Embase databases were searched using a validated search filter for prediction model studies. Two reviewers independently assessed studies for inclusion and risk of bias. Measures of model performance (discrimination and calibration) to assess the risk of HCC at specified time points were identified. A random effects meta-analysis was performed on a subset of studies that reported performance of the same model.
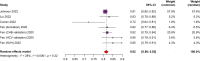
Results: A total of 7,854 studies were identified. After review, 14 studies with a total of 94,014 participants were included; 45% of patients had viral hepatitis, 27% ALD (alcohol-related liver disease) and 19% MASLD (metabolic dysfunction-associated steatotic liver disease). Follow-up ranged from 15.1-138 months. Only one model was developed using a competing risk approach. Age (7 models) and sex (6 models) were the most frequently included predictors. Model discrimination (AUROC or c-statistic) ranged from 0.61-0.947. Only the 'aMAP' score (age, male sex, albumin, bilirubin, and platelets) had sufficient external validation for quantitative analysis, with a pooled c-statistic of 0.81 (95% CI 0.80-0.83). Calibration was reported in only 9 of 14 studies. All studies were rated at high risk of bias.
Conclusion: Studies describing risk prediction of HCC in non-viral chronic liver disease are poorly reported, have a high risk of bias and do not account for competing risk events. Patients with ALD and MASLD are underrepresented in development and validation cohorts. These factors remain barriers to the clinical utility and uptake of HCC risk models for those with the most common liver diseases.
Impact and implications: The recent EASL policy statement emphasises the potential of risk-based surveillance to reduce both hepatocellular carcinoma (HCC)-related deaths and surveillance costs. This study addresses the gap in understanding the performance of current HCC risk models in patients with non-viral liver diseases, reflecting the epidemiological landscape of liver disease in Western countries. In our review of these models we identified several key concerns regarding reporting standards and risk of bias and confirmed that patients with alcohol-related liver disease and metabolic dysfunction-associated steatotic liver disease are underrepresented in model development and validation cohorts. Additionally, most models fail to account for the significant risk of competing events, leading to potential overestimation of true HCC risk. This study highlights these critical issues that may hinder the implementation of risk models in clinical practice and offers key recommendations for future model development studies.
Keywords: Carcinoma; Hepatocellular; Liver Cirrhosis; Meta-analysis; Systematic review.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest that pertain to this work. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures
References
-
- Rahib L., Smith B.D., Aizenberg R., et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. - PubMed
-
- Llovet J.M., Kelley R.K., Villanueva A., et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. - PubMed
-
- Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous