Effect of calcium ions on the aggregation of highly phosphorylated tau
- PMID: 39655264
- PMCID: PMC11626071
- DOI: 10.1016/j.bbrep.2024.101887
Effect of calcium ions on the aggregation of highly phosphorylated tau
Abstract
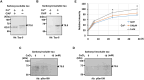
Tau is typically an axonal protein, but in neurons of brains affected by Alzheimer's disease (AD), aggregation of hyperphosphorylated tau in the somatodendritic compartment causes neuronal death. We have previously demonstrated that tau mRNA is transported within dendrites and undergoes immediate translation and hyperphosphorylation of AD epitopes in response to NMDA receptor stimulation. Although this explains the emergence of hyperphosphorylated tau in dendrites, the relationship between tau hyperphosphorylation and aggregation is not well understood. In this study, we found that recombinant highly phosphorylated tau purified from NG108-15 rodent neuroblastoma/glioma cells transfected with both tau and GSK3β expression vectors bound calcium ions and formed sarkosyl-insoluble aggregates. In addition, thioflavin T analysis revealed that this highly phosphorylated tau tended to aggregate on its own, further facilitated by calcium ions. When NG108-15 cells expressing the highly phosphorylated tau were treated with calcium ionophore, sarkosyl-insoluble tau was generated. Interestingly, these cells exhibited resistance to both calcium ionophore-induced cytotoxicity and glutamate-induced excitotoxicity. We further found that sarkosyl-insoluble phosphorylated tau was increased in cultured hippocampal neurons due to glutamate-induced hyperactivity. Our data suggest that hyperphosphorylated tau synthesized in response to NMDA receptor stimulation contributes to regulation of neuronal activity by binding calcium ions, but that this calcium binding may cause tau to adopt an aggregated form.
Keywords: Aggregation; Calcium; Excitotoxicity; Highly phosphorylation; Tau.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
LinkOut - more resources
Full Text Sources

