Delphinidin induces a fast-to-slow muscle fiber type shift through the AMPK signaling pathway in C2C12 myotubes
- PMID: 39655265
- PMCID: PMC11626064
- DOI: 10.1016/j.bbrep.2024.101884
Delphinidin induces a fast-to-slow muscle fiber type shift through the AMPK signaling pathway in C2C12 myotubes
Abstract
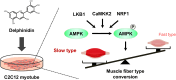
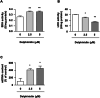
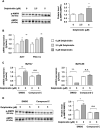
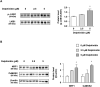
Delphinidin, a plant anthocyanidin, suppresses disuse muscle atrophy in mice. However, its effect on muscle fiber type shift is unclear. To examine whether delphinidin affects skeletal muscle fiber type, differentiated C2C12 cells were treated with delphinidin. Results revealed that delphinidin upregulated the mRNA expression of myosin heavy chain type I (MyHCI), troponin C1, troponin I1, and MyHCIIx and increased slow MyHC protein level in C2C12 myotubes. Delphinidin also enhanced succinic dehydrogenase (SDH) activities and suppressed lactate dehydrogenase (LDH) activity. Adenosine monophosphate-activated protein kinase (AMPK) inhibition attenuated delphinidin-induced MyHCI upregulation and MyHCIIb downregulation. We investigated the effect of delphinidin on the upstream factors involved in AMPK activation. Delphinidin increased liver kinase B1 (LKB1) phosphorylation and nuclear respiratory factor 1 (NRF1) and calcium/calmodulin-dependent protein kinase 2 (CaMKK2) protein levels. In conclusion, delphinidin induced muscle fiber type conversion from fast-twitch to slow-twitch muscles through the AMPK signaling pathway.
Keywords: AMPK; Anthocyanidin; C2C12 myotube; MyHC.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
LinkOut - more resources
Full Text Sources

