Inositol 1,4,5-Trisphosphate Receptor 1 Gain-of-Function Increases the Risk for Cardiac Arrhythmias in Mice and Humans
- PMID: 39655431
- PMCID: PMC11932448
- DOI: 10.1161/CIRCULATIONAHA.124.070563
Inositol 1,4,5-Trisphosphate Receptor 1 Gain-of-Function Increases the Risk for Cardiac Arrhythmias in Mice and Humans
Abstract
Background: Ca2+ mishandling in cardiac Purkinje cells is a well-known cause of cardiac arrhythmias. The Purkinje cell resident inositol 1,4,5-trisphosphate receptor 1 (ITPR1) is believed to play an important role in Ca2+ handling, and ITPR1 gain-of-function (GOF) has been implicated in cardiac arrhythmias. However, nearly all known disease-associated ITPR1 variants are loss-of-function and are primarily linked to neurological disorders. Whether ITPR1 GOF has pathological consequences, such as cardiac arrhythmias, is unclear. This study aimed to identify human ITPR1 GOF variants and determine the impact of ITPR1 GOF on Ca2+ handling and arrhythmia susceptibility.
Methods: There are a large number of rare ITPR1 missense variants reported in open data repositories. Based on their locations in the ITPR1 channel structure, we selected and characterized 33 human ITPR1 missense variants from open databases and identified 21 human ITPR1 GOF variants. We generated a mouse model carrying a human ITPR1 GOF variant, ITPR1-W1457G (W1447G in mice).
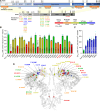
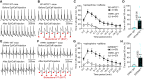
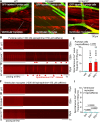
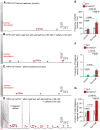
Results: We showed that the ITPR1-W1447G+/- and recently reported ITPR1-D2594K+/- GOF mutant mice were susceptible to stress-induced ventricular arrhythmias. Confocal Ca2+ and voltage imaging in situ in heart slices and Ca2+ imaging and patch-clamp recordings of isolated Purkinje cells showed that ITPR1-W1447G+/- and ITPR1-D2594K+/- variants increased the occurrence of stress-induced spontaneous Ca2+ release, delayed afterdepolarization, and triggered activity in Purkinje cells. To assess the potential role of ITPR1 variants in arrhythmia susceptibility in humans, we looked up a gene-based association study in the UK Biobank data set and identified 7 rare ITPR1 missense variants showing potential association with cardiac arrhythmias. Remarkably, in vitro functional characterization revealed that all these 7 ITPR1 variants resulted in GOF.
Conclusions: Our studies in mice and humans reveal that enhanced function of ITPR1, a well-known movement disorder gene, increases the risk for cardiac arrhythmias.
Keywords: Purkinje cells; cardiac ryanodine receptor; inositol 1,4,5-trisphosphate receptor; sarcoplasmic reticulum; spontaneous Ca2+ release; triggered activity; ventricular arrhythmias.
Conflict of interest statement
None.
Figures




References
-
- Patel S, Joseph SK, Thomas AP. Molecular properties of inositol 1,4,5-trisphosphate receptors. Cell Calcium. 1999;25:247–264. doi: 10.1054/ceca.1999.0021 - PubMed
-
- Stathopulos PB, Seo MD, Enomoto M, Amador FJ, Ishiyama N, Ikura M. Themes and variations in ER/SR calcium release channels: structure and function. Physiology (Bethesda). 2012;27:331–342. doi: 10.1152/physiol.00013.2012 - PubMed
-
- Parys JB, De Smedt H. Inositol 1,4,5-trisphosphate and its receptors. Adv Exp Med Biol. 2012;740:255–279. doi: 10.1007/978-94-007-2888-2_11 - PubMed
-
- Berridge MJ. The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol Rev. 2016;96:1261–1296. doi: 10.1152/physrev.00006.2016 - PubMed
-
- Berridge MJ. Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta. 2009;1793:933–940. doi: 10.1016/j.bbamcr.2008.10.005 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

