Neuropathology of trisomy 21 mosaicism in a case with early-onset dementia
- PMID: 39655579
- PMCID: PMC11772706
- DOI: 10.1002/alz.14394
Neuropathology of trisomy 21 mosaicism in a case with early-onset dementia
Abstract
Introduction: This study investigated the impact of trisomy 21 mosaicism (mT21) on Alzheimer's disease (AD) neuropathology in a well-characterized clinical case described by Ringman et al.
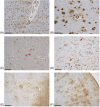
Methods: We describe AD neuropathology in mT21 including amyloid beta, phosphorylated tau, astrogliosis, microgliosis, α-synuclein, and TAR DNA-binding protein 43 (TDP-43) in cerebral cortex, hippocampal subregions, and amygdala using immunohistochemistry.
Results: We observed high AD neuropathologic change with a score of A3B3C3. In addition, there was widespread astrogliosis, cerebral amyloid angiopathy, and perivascular space widening throughout the brain. Lewy bodies and neurites were noted in the amygdala only and no TDP-43 was observed.
Discussion: The findings in this case report highlight that mT21 is sufficient to induce AD neuropathology and early-onset dementia.
Highlights: Trisomy 21 mosaicism (mT21) occurs when three copies of chromosome 21 are present in some but not all somatic cells in an individual. mT21 accounts for ≈ 2% of people diagnosed with Down syndrome (DS). Immunohistochemical identification of amyloid beta, tau, astrocytes, microglia, α-synuclein, and TAR DNA-binding protein 43 show that Alzheimer's disease (AD) pathology in mT21 is similar to full trisomy 21. The findings in this case report highlight that mT21 is sufficient to induce AD neuropathology and early-onset dementia.
Keywords: Alzheimer's disease; Down syndrome; amyloid beta; neurofibrillary tangles; perivascular space widening.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare there are no competing interests. Author disclosures are available in the supporting information.
Figures


References
-
- Down JL. Observations on an ethnic classification of idiots. 1866. Ment Retard. 1995;33(1):54‐56. - PubMed
-
- Shin M, Siffel C, Correa A. Survival of children with mosaic Down syndrome. Am J Med Genet A. 2010;152A(3):800‐801. - PubMed
-
- Papavassiliou P, Charalsawadi C, Rafferty K, Jackson‐Cook C. Mosaicism for trisomy 21: a review. Am J Med Genet A. 2015;167A(1):26‐39. - PubMed
-
- Rumble B, Retallack R, Hilbich C, et al. Amyloid A4 protein and its precursor in Down's syndrome and Alzheimer's disease. N Engl J Med. 1989;320(22):1446‐1452. - PubMed

