Pharmacological target sites for restoration of age-associated deficits in NMDA receptor-mediated norepinephrine release in brain
- PMID: 39655655
- PMCID: PMC11629444
- DOI: 10.1111/jnc.16280
Pharmacological target sites for restoration of age-associated deficits in NMDA receptor-mediated norepinephrine release in brain
Abstract
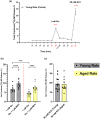
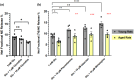
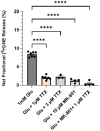
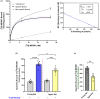
Aging affects virtually all organs of the body, but perhaps it has the most profound effects on the brain and its neurotransmitter systems, which influence a wide range of crucial functions, such as attention, focus, mood, neuroendocrine and autonomic functions, and sleep cycles. All of these essential functions, as well as fundamental cognitive processes such as memory, recall, and processing speed, utilize neuronal circuits that depend on neurotransmitter signaling between neurons. Glutamate (Glu), the main excitatory neurotransmitter in the CNS, is involved in most neuronal excitatory functions, including release of the neurotransmitter norepinephrine (NE). Previous studies from our lab demonstrated that the age-associated decline in Glu-stimulated NE release in rat cerebral cortex and hippocampus mediated by NMDA glutamate receptors, as well as deficits in dendritic spines, and cognitive functions are fully rescued by the CNS stimulant amphetamine. Here we further investigated Glu-stimulated NE release in the cerebral cortex to identify additional novel target sites for restoration of Glu-stimulated NE release. We found that blockade of alpha-2 adrenergic receptors fully restores Glu-stimulated NE release to the levels of young controls. In addition, we investigated the density and responsiveness of NMDA receptors as a potential underlying neuronal mechanism that could account for the observed age-associated decline in Glu-stimulated NE release. In the basal state of the receptor (no added glutamate and glycine) the density of NMDA receptors in the cortex from young and aged rats was similar. However, in contrast, in the presence of 10 μM added glutamate, which opens the receptor channel and increases the number of available [3H]-MK-801 binding sites within the channel, the density of [3H]-MK-801 binding sites was significantly less in the cortex from aged rats.
Keywords: NMDA receptors; aging; alpha‐2‐adrenergic receptors; norepinephrine release.
© 2024 The Author(s). Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Conflict of interest statement
The authors declare that the research was directed without commercial or financial relationships that could be interpreted as a potential conflict of interest.
Figures


Similar articles
-
Restoration of norepinephrine release, cognitive performance, and dendritic spines by amphetamine in aged rat brain.Aging Cell. 2024 Apr;23(4):e14087. doi: 10.1111/acel.14087. Epub 2024 Feb 8. Aging Cell. 2024. PMID: 38332648 Free PMC article.
-
N-methyl-D-aspartic acid (NMDA) and non-NMDA receptors regulating hippocampal norepinephrine release. I. Location on axon terminals and pharmacological characterization.J Pharmacol Exp Ther. 1992 Jan;260(1):232-7. J Pharmacol Exp Ther. 1992. PMID: 1370540
-
N-methyl-D-aspartic acid (NMDA) and non-NMDA receptors regulating hippocampal norepinephrine release. III. Changes in the NMDA receptor complex induced by their functional cooperation.J Pharmacol Exp Ther. 1992 Oct;263(1):327-33. J Pharmacol Exp Ther. 1992. PMID: 1357159
-
Glycine binding sites of presynaptic NMDA receptors may tonically regulate glutamate release in the rat visual cortex.J Neurophysiol. 2007 Jan;97(1):817-23. doi: 10.1152/jn.00980.2006. Epub 2006 Nov 8. J Neurophysiol. 2007. PMID: 17093111
-
Brain free magnesium homeostasis as a target for reducing cognitive aging.In: Vink R, Nechifor M, editors. Magnesium in the Central Nervous System [Internet]. Adelaide (AU): University of Adelaide Press; 2011. In: Vink R, Nechifor M, editors. Magnesium in the Central Nervous System [Internet]. Adelaide (AU): University of Adelaide Press; 2011. PMID: 29920011 Free Books & Documents. Review.
References
-
- Bettencourt, J. W. , McLaury, A. R. , Limberg, A. K. , Vargas‐Hernandez, J. S. , Bayram, B. , Owen, A. R. , Berry, D. J. , Sanchez‐Sotelo, J. , Morrey, M. E. , van Wijnen, A. J. , & Abdel, M. P. (2020). Total protein staining is superior to classical or tissue‐specific protein staining for standardization of protein biomarkers in heterogeneous tissue samples. Gene Rep, 19, 1–7. 10.1016/j.genrep.2020.100641 - DOI - PMC - PubMed
-
- Carter, A. J. (1997). Hippocampal noradrenaline release in awake, freely moving rats is regulated by alpha‐2 adrenoceptors but not by adenosine receptors. The Journal of Pharmacology and Experimental Therapeutics, 281(2), 648–654. https://www.ncbi.nlm.nih.gov/pubmed/9152369 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

