Performance of the WID-qEC test to detect uterine cancers in black women with abnormal uterine bleeding: A prospective observational cohort study in Ghana
- PMID: 39655721
- PMCID: PMC11701417
- DOI: 10.1002/ijc.35260
Performance of the WID-qEC test to detect uterine cancers in black women with abnormal uterine bleeding: A prospective observational cohort study in Ghana
Abstract
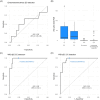
The burden of uterine cancer is growing and, in the US and UK, mortality rates are poorest among black women. Early detection of these cancers is critical and poor performance of ultrasound in black women may contribute to adverse outcomes. Limited data on this topic are available from Africa. We assessed whether a simple DNA methylation test, the WID-qEC, enables detection of all epithelial uterine (endometrial and cervical) cancers in women presenting with abnormal uterine bleeding (AUB) in Ghana. Among 118 women ≥40 years presenting with AUB, 106 consented to the study and a cervicovaginal sample was obtained for WID-qEC testing. Subsequent to ultrasound assessment 102 women had a cervical or endometrial biopsy. Histopathology, ultrasound and WID-qEC testing were analyzed and compared. Among the 102 volunteers, 8 and 15 were diagnosed with endometrial and cervical cancer (EC and CC), respectively. The receiver operating characteristic (ROC) area under the curve (AUC) was 0.56 (95% confidence interval [CI] 0.25-0.86) for sonographic endometrial thickness (ET) and 0.98 (95% CI 0.94-1.00) for the WID-qEC test. Sensitivity and specificity of the prespecified ET ≥5 mm were 66.7% (95% CI 24.1-94.0) and 22.7 (95% CI 12.0-38.2) and for the prespecified WID-qEC SUM-PMR ≥ 0.3 were 100% (95% CI 56.1-100.0) and 76.1 (96%CI 60.9-86.9), respectively. In addition, 15 CCs were detected by the WID-qEC test [sensitivity 100% (95% CI 74.7-100.0)]. The WID-qEC test accurately detects both EC and CC in black women presenting with AUB.
Keywords: DNA methylation; abnormal bleeding; black women; endometrial cancer; ultrasound.
© 2024 The Author(s). International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Conflict of interest statement
J.E.B., C.H., E.R., A.J., I.E. and M.W. are inventors on WID‐qEC related patent applications. J.E.B., C.H. and M.W. are shareholders of Sola Diagnostics GmbH which holds own and licensed IP protecting commercialization of the WID‐qEC test. E.R. is an employee of Sola Diagnostics GmbH. The other authors have no conflict of interest to declare.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

