Lentiviral Gene Therapy with CD34+ Hematopoietic Cells for Hemophilia A
- PMID: 39655790
- PMCID: PMC11875532
- DOI: 10.1056/NEJMoa2410597
Lentiviral Gene Therapy with CD34+ Hematopoietic Cells for Hemophilia A
Abstract
Background: Severe hemophilia A is managed with factor VIII replacement or hemostatic products that stop or prevent bleeding. Data on gene therapy with hematopoietic stem-cell (HSC)-based expression of factor VIII for the treatment of severe hemophilia A are lacking.
Methods: We conducted a single-center study involving five participants 22 to 41 years of age with severe hemophilia A without factor VIII inhibitors. Autologous HSCs were transduced with CD68-ET3-LV - a lentiviral vector including a new F8 transgene (ET3) with a myeloid-directed CD68 promoter - either without transduction enhancer (group 1) or with transduction enhancer (group 2). Transduced HSCs were transplanted into recipients after myeloablative conditioning. The treatment was assessed for safety (engraftment and regimen-related toxic effects) and efficacy (factor VIII activity and annualized bleeding rate).
Results: Participants received CD68-ET3-LV-transduced autologous CD34+ HSCs at doses of 5.0×106 to 6.1×106 per kilogram of body weight. The vector copy numbers in the final drug product were 1.0 and 0.6 copies per cell for the two participants in group 1 and 1.5, 0.6, and 2.2 copies per cell for the three participants in group 2. The duration of severe neutropenia was 7 to 11 days and of severe thrombocytopenia was 1 to 7 days. The median factor VIII activity level, measured with the use of a one-stage assay, after day 28 until the last follow-up visit was 5.2 IU per deciliter (range, 3.0 to 8.7) and 1.7 IU per deciliter (range, 1.0 to 4.0) with a peripheral-blood vector copy number of 0.2 and 0.1 copies per cell, respectively, in the two group 1 participants, and 37.1 IU per deciliter (range, 18.3 to 73.6), 19.3 IU per deciliter (range, 6.6 to 34.5), and 39.9 IU per deciliter (range, 20.6 to 55.1) with a peripheral-blood vector copy number of 4.4, 3.2, and 4.8 copies per cell, respectively, in the three group 2 participants. The annualized bleeding rate was zero for all five participants over a cumulative follow-up of 81 months (median follow-up, 14 months; range, 9 to 27).
Conclusions: Gene therapy for hemophilia A with the use of lentiviral vector-transduced autologous HSCs resulted in stable factor VIII expression, with factor VIII activity correlating to vector copy number in the peripheral blood. (Funded by the Ministry of Science and Technology, Government of India, and others; ClinicalTrials.gov number, NCT05265767; Clinical Trials Registry-India number, CTRI/2022/03/041304.).
Copyright © 2024 Massachusetts Medical Society.
Figures

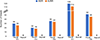
Comment in
-
Lentiviral Gene Therapy with CD34+ Hematopoietic Cells for Hemophilia A.N Engl J Med. 2025 May 1;392(17):1765. doi: 10.1056/NEJMc2502741. N Engl J Med. 2025. PMID: 40305727 No abstract available.
-
Lentiviral Gene Therapy with CD34+ Hematopoietic Cells for Hemophilia A.N Engl J Med. 2025 May 1;392(17):1765-1766. doi: 10.1056/NEJMc2502741. N Engl J Med. 2025. PMID: 40305728 No abstract available.
-
Lentiviral Gene Therapy with CD34+ Hematopoietic Cells for Hemophilia A. Reply.N Engl J Med. 2025 May 1;392(17):1766. doi: 10.1056/NEJMc2502741. N Engl J Med. 2025. PMID: 40305729 No abstract available.
References
-
- Srivastava A, Santagostino E, Dougall A, Kitchen S, Sutherland M, Pipe SW, et al. WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia : the official journal of the World Federation of Hemophilia. 2020. Aug;26 Suppl 6:1–158. - PubMed
-
- Pierce GF, Fong S, Long BR, Kaczmarek R. Deciphering conundrums of adeno-associated virus liver-directed gene therapy: focus on hemophilia. Journal of thrombosis and haemostasis : JTH. 2024. May;22(5):1263–89. - PubMed
-
- High KH, Nathwani A, Spencer T, Lillicrap D. Current status of haemophilia gene therapy. Haemophilia : the official journal of the World Federation of Hemophilia. 2014. May;20 Suppl 4:43–9. - PubMed
-
- Ozelo MC, Mahlangu J, Pasi KJ, Giermasz A, Leavitt AD, Laffan M, et al. Valoctocogene Roxaparvovec Gene Therapy for Hemophilia A. The New England journal of medicine. 2022. Mar 17;386(11):1013–25. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
