Tissue-resident natural killer cells support survival in pancreatic cancer through promotion of cDC1-CD8 T activity
- PMID: 39656086
- PMCID: PMC11630822
- DOI: 10.7554/eLife.92672
Tissue-resident natural killer cells support survival in pancreatic cancer through promotion of cDC1-CD8 T activity
Abstract
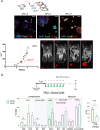
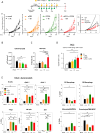
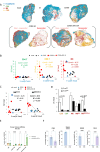
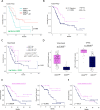
The immunosuppressive microenvironment in pancreatic ductal adenocarcinoma (PDAC) prevents tumor control and strategies to restore anti-cancer immunity (i.e. by increasing CD8 T-cell activity) have had limited success. Here, we demonstrate how inducing localized physical damage using ionizing radiation (IR) unmasks the benefit of immunotherapy by increasing tissue-resident natural killer (trNK) cells that support CD8 T activity. Our data confirms that targeting mouse orthotopic PDAC tumors with IR together with CCR5 inhibition and PD1 blockade reduces E-cadherin positive tumor cells by recruiting a hypoactive NKG2D-ve NK population, phenotypically reminiscent of trNK cells, that supports CD8 T-cell involvement. We show an equivalent population in human single-cell RNA sequencing (scRNA-seq) PDAC cohorts that represents immunomodulatory trNK cells that could similarly support CD8 T-cell levels in a cDC1-dependent manner. Importantly, a trNK signature associates with survival in PDAC and other solid malignancies revealing a potential beneficial role for trNK in improving adaptive anti-tumor responses and supporting CCR5 inhibitor (CCR5i)/αPD1 and IR-induced damage as a novel therapeutic approach.
Keywords: KPC; NK cells; cancer biology; human; immune; immunology; inflammation; mouse; pancreatic; tissue resident.
© 2024, Go, Demetriou, Valenzano et al.
Conflict of interest statement
SG, CD, GV, SH, SL, HF, EA, SS, RB, MM, SM, JM, KJ, EN No competing interests declared
Figures












Update of
- doi: 10.1101/2023.10.14.562332
- doi: 10.7554/eLife.92672.1
- doi: 10.7554/eLife.92672.2
References
-
- Ajuebor MN, Wondimu Z, Hogaboam CM, Le T, Proudfoot AEI, Swain MG. CCR5 deficiency drives enhanced natural killer cell trafficking to and activation within the liver in murine T cell-mediated hepatitis. The American Journal of Pathology. 2007;170:1975–1988. doi: 10.2353/ajpath.2007.060690. - DOI - PMC - PubMed
-
- Alshetaiwi H, Pervolarakis N, McIntyre LL, Ma D, Nguyen Q, Rath JA, Nee K, Hernandez G, Evans K, Torosian L, Silva A, Walsh C, Kessenbrock K. Defining the emergence of myeloid-derived suppressor cells in breast cancer using single-cell transcriptomics. Science Immunology. 2020;5:eaay6017. doi: 10.1126/sciimmunol.aay6017. - DOI - PMC - PubMed
-
- Barry KC, Hsu J, Broz ML, Cueto FJ, Binnewies M, Combes AJ, Nelson AE, Loo K, Kumar R, Rosenblum MD, Alvarado MD, Wolf DM, Bogunovic D, Bhardwaj N, Daud AI, Ha PK, Ryan WR, Pollack JL, Samad B, Asthana S, Chan V, Krummel MF. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nature Medicine. 2018;24:1178–1191. doi: 10.1038/s41591-018-0085-8. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

