Decreased urinary excretion of norepinephrine and dopamine in autonomic synucleinopathies
- PMID: 39656385
- PMCID: PMC12000174
- DOI: 10.1007/s10286-024-01093-6
Decreased urinary excretion of norepinephrine and dopamine in autonomic synucleinopathies
Abstract
Background: Autonomic synucleinopathies feature autonomic failure and intracellular deposition of the protein alpha-synuclein. Three such conditions are the Lewy body diseases (LBDs) Parkinson's disease (PD) and pure autonomic failure (PAF) and the non-LBD synucleinopathy multiple system atrophy (MSA). These diseases all entail catecholaminergic abnormalities in the brain, sympathetically innervated organs, or both; however, little is known about renal catecholaminergic functions in autonomic synucleinopathies. We measured urinary excretion rates of the sympathetic neurotransmitter norepinephrine, the hormone epinephrine, the autocrine-paracrine substance dopamine, the catecholamine precursor 3,4-dihydroxyphenylalanine (DOPA), 3,4-dihydroxyphenylglycol (DHPG, the main neuronal metabolite of norepinephrine), and 3,4-dihydroxyphenylacetic acid (DOPAC, a major dopamine metabolite), in PD, PAF, and MSA groups and controls.
Methods: Data were reviewed from all research participants who had urine collections (usually 3.5 h) at the National Institutes of Health (NIH) Clinical Center from 1995 to 2024. The control cohort had neither autonomic failure nor a movement disorder.
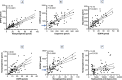
Results: Norepinephrine excretion rates were decreased compared with controls in PD (p = 0.0001), PAF (p < 0.0001), and MSA (p < 0.0001). Dopamine excretion was also decreased in the three groups (PD: p = 0.0136, PAF: p = 0.0027, MSA: p = 0.0344). DHPG excretion was decreased in PD (p = 0.0004) and PAF (p = 0.0004) but not in MSA. DOPA and epinephrine excretion did not differ among the study groups.
Conclusions: Autonomic synucleinopathies involve decreased urinary excretion rates of norepinephrine and dopamine. Since virtually all of urinary dopamine in humans is derived from circulating DOPA, the low rates of urinary norepinephrine and dopamine excretion may reflect dysfunctions in the renal sympathetic noradrenergic system, the DOPA-dopamine autocrine-paracrine system, or both systems.
Keywords: Autonomic; Dopamine; Kidney; Norepinephrine; Synuclein.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Figures


References
-
- Bannister R (1993) Multiple-system atrophy and pure autonomic failure. In: Low PA (ed) Clinical autonomic disorders. Little, Brown and Company, Boston, pp 517–535
-
- Bannister R, Oppenheimer DR (1972) Degenerative diseases of the nervous system associated with autonomic failure. Brain 95:457–474 - PubMed
-
- Barbeau A (1967) The “pink spot”, 3,4-dimethoxyphenylethylamine and dopamine. Relationship to Parkinson’s disease and to schizophrenia. Rev Can Biol 26:55–79 - PubMed
-
- Barbeau A, Murphy GF, Sourkes TL (1961) Excretion of dopamine in diseases of Basal Ganglia. Science 133:1706–1707 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

