Characterization of PSA dynamics and oncological outcomes in patients with metastatic hormone-sensitive prostate cancer treated with androgen receptor signaling inhibitors
- PMID: 39656401
- PMCID: PMC11842405
- DOI: 10.1007/s10147-024-02676-z
Characterization of PSA dynamics and oncological outcomes in patients with metastatic hormone-sensitive prostate cancer treated with androgen receptor signaling inhibitors
Abstract
Background: This study investigated the characteristics of prostate-specific antigen (PSA) dynamics when androgen receptor signaling inhibitor (ARSI), or vintage agent (bicalutamide) was used for patients with metastatic hormone-sensitive prostate cancer (mHSPC).
Patients and methods: A total of 213 mHSPC patients from each of the ARSI and bicalutamide groups treated between 2015 and 2022 were selected from multiple institutions using propensity score-matched analysis to align backgrounds. PSA progression-free survival (PFS) and overall survival (OS) were assessed. PSA level at 3 months, PSA nadir level, and time to PSA nadir were examined to analyze of PSA kinetics.
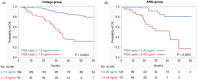
Results: ARSI treatment significantly improved PSA PFS compared to bicalutamide (P = 0.0063), although no significant difference in OS was seen (P = 0.3134). No significant differences were observed between treatment groups in median PSA levels at 3 months (1.47 vs 0.52 ng/ml, P = 0.3042) or PSA nadir levels (0.263 vs 0.1345 ng/ml, P = 0.1228). Bicalutamide treatment demonstrated longer time to nadir than ARSI in progression-free cases (median: 243 vs 213.5 days, P = 0.0003). Survival tree analysis found that PSA nadir ≤ 1.5 ng/ml and time to nadir ≥ 145 days were the optimal cut-offs for best stratifying OS with bicalutamide, while PSA nadir ≤ 0.45 ng/ml and time to nadir ≥ 70 days were optimal with ARSI.
Conclusion: No significant differences in PSA response was seen between groups; however, distinct optimal cut-offs were demonstrated for PSA nadir and time to nadir. The present findings will be useful for optimal PSA monitoring for mHSPC patients and for early identification of poor-prognosis populations.
Keywords: Androgen receptor signaling inhibitor; Bicalutamide; Metastatic hormone-sensitive prostate cancer; PSA dynamics; PSA nadir; Time to PSA nadir.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no conflict of interest. Ethical approval: The protocol for this research project has been approved by a suitably constituted ethics committee of the institution, and it conforms to the provisions of the Declaration of Helsinki. This study was approved by the Institutional Review Board of Chiba University Hospital (M10238) and Osaka Medical and Pharmaceutical University Hospital (RIN750-2571).
Figures



Similar articles
-
Clinical benefits of androgen receptor signaling inhibitors in patients with metastatic hormone-sensitive prostate cancer: real-world data from a multi-center study.Jpn J Clin Oncol. 2025 Aug 3;55(8):954-962. doi: 10.1093/jjco/hyaf079. Jpn J Clin Oncol. 2025. PMID: 40382671
-
A retrospective study of prognostic factors and prostate-specific antigen dynamics in Japanese patients with metastatic hormone-sensitive prostate cancer who received combined androgen blockade therapy with bicalutamide.Int J Clin Oncol. 2024 Oct;29(10):1564-1573. doi: 10.1007/s10147-024-02597-x. Epub 2024 Aug 17. Int J Clin Oncol. 2024. PMID: 39153094 Free PMC article.
-
Efficacy of androgen receptor signaling inhibitors in combination with androgen deprivation therapy for castration-sensitive metastatic prostate cancer: a retrospective analysis in a Japanese cohort.Int J Clin Oncol. 2025 Feb;30(2):351-357. doi: 10.1007/s10147-024-02670-5. Epub 2024 Dec 18. Int J Clin Oncol. 2025. PMID: 39692835 Free PMC article.
-
International study into the use of intermittent hormone therapy in the treatment of carcinoma of the prostate: a meta-analysis of 1446 patients.BJU Int. 2007 May;99(5):1056-65. doi: 10.1111/j.1464-410X.2007.06770.x. Epub 2007 Mar 6. BJU Int. 2007. PMID: 17346277 Review.
-
Selective treatment de-escalation in advanced prostate cancer: have we come full circle?BJU Int. 2025 May;135(5):733-740. doi: 10.1111/bju.16632. Epub 2025 Jan 2. BJU Int. 2025. PMID: 39748463 Review.
References
-
- Siegel RL, Giaquinto AN, Jemal A (2024) Cancer statistics, 2024. CA Cancer J Clin 74(1):12–49. 10.3322/caac.21820 - PubMed
-
- Davis ID, Martin AJ, Stockler MR et al (2019) Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med 381(2):121–131. 10.1056/NEJMoa1903835 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

