Advances in dorzolamide hydrochloride delivery: harnessing nanotechnology for enhanced ocular drug delivery in glaucoma management
- PMID: 39656411
- PMCID: PMC11631835
- DOI: 10.1186/s11671-024-04154-x
Advances in dorzolamide hydrochloride delivery: harnessing nanotechnology for enhanced ocular drug delivery in glaucoma management
Abstract
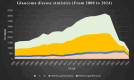
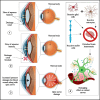
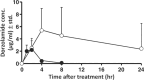
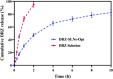
Dorzolamide hydrochloride (DRZ) is a carbonic anhydrase inhibitor utilized in managing elevated intraocular pressure (IOP) associated with glaucoma. However, its clinical effectiveness is hindered by a short half-life, low residence time, and the need for frequent dosing, highlighting the necessity for innovative delivery systems. This work reviews recent advancements in DRZ delivery, particularly focusing on cyclodextrin complexation and nanotechnology applications. It explores the potential of cyclodextrin derivatives to enhance DRZ's bioavailability. DRZ cyclodextrin complexes or nanoparticulate systems maintain high drug concentrations in the eye while minimizing irritation and viscosity-related issues. Nanotechnology introduces nanoparticle-based carriers such as polymeric nanoparticles, solid lipid nanoparticles, liposomes, niosomes, and nanoemulsions. These formulations enable sustained drug release, improved corneal permeation, and enhanced patient compliance. Clinical trials have shown that DRZ nanoparticle eye drops and nanoliposome formulations offer efficacy comparable to conventional therapies, with the potential for better tolerability. Overall, this review highlights significant progress in DRZ delivery systems, suggesting their potential to transform glaucoma treatment by addressing current limitations and improving therapeutic outcomes.
Keywords: Cyclodextrin complexation; Dorzolamide hydrochloride; Drug delivery; Glaucoma; Nanotechnology.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Ocular Dorzolamide Nanoliposomes for Prolonged IOP Reduction: in-vitroand in-vivo Evaluation in Rabbits.Iran J Pharm Res. 2016 Winter;15(1):205-12. Iran J Pharm Res. 2016. PMID: 27610160 Free PMC article.
-
Technological Advances in a Therapy of Primary Open-Angle Glaucoma: Insights into Current Nanotechnologies.J Clin Med. 2023 Sep 6;12(18):5798. doi: 10.3390/jcm12185798. J Clin Med. 2023. PMID: 37762739 Free PMC article. Review.
-
Nanoemulsion as a potential ophthalmic delivery system for dorzolamide hydrochloride.AAPS PharmSciTech. 2009;10(3):808-19. doi: 10.1208/s12249-009-9268-4. Epub 2009 Jun 18. AAPS PharmSciTech. 2009. PMID: 19536653 Free PMC article.
-
Leucaena leucocephala (Lam.) galactomannan nanoparticles: Optimization and characterization for ocular delivery in glaucoma treatment.Int J Biol Macromol. 2019 Oct 15;139:1252-1262. doi: 10.1016/j.ijbiomac.2019.08.107. Epub 2019 Aug 13. Int J Biol Macromol. 2019. PMID: 31419555
-
Applications of Nanotechnology-mediated Herbal Nanosystems for Ophthalmic Drug.Pharm Nanotechnol. 2024;12(3):229-250. doi: 10.2174/2211738511666230816090046. Pharm Nanotechnol. 2024. PMID: 37587812 Review.
References
-
- Loftsson T, Jansook P, Stefánsson E. Topical drug delivery to the eye: dorzolamide. Acta Ophthalmol. 2012;90:603–8. 10.1111/j.1755-3768.2011.02299.x. - PubMed
-
- Tribble JR, Hui F, Quintero H, et al. Neuroprotection in glaucoma: mechanisms beyond intraocular pressure lowering. Mol Aspects Med. 2023;92:101193. 10.1016/j.mam.2023.101193. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
