S-Nitrosylation of NOTCH1 Regulates Mesenchymal Stem Cells Differentiation Into Hepatocyte-Like Cells by Inhibiting Notch Signalling Pathway
- PMID: 39656437
- PMCID: PMC11629812
- DOI: 10.1111/jcmm.70274
S-Nitrosylation of NOTCH1 Regulates Mesenchymal Stem Cells Differentiation Into Hepatocyte-Like Cells by Inhibiting Notch Signalling Pathway
Abstract
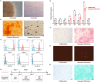
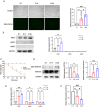
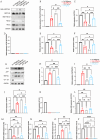
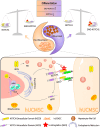
The differentiation of mesenchymal stem cells (MSCs) into hepatocyte-like cells (HLCs) is considered one of the most promising strategies for alternative hepatocyte transplantation to treat end-stage liver disease. To advance this method, it is crucial to gain a deeper understanding of the mechanisms governing hepatogenic differentiation. The study demonstrated that suppression of the intracellular domain release of the Notch pathway receptor via the γ-secretase inhibitor N-[(3, 5-difluorophenyl)acetyl]-L-alanyl-2-phenylglycine-1, 1-dimethylethyl ester (DAPT) significantly promotes the expression of hepatocyte-related genes and proteins in HLCs. Increased expression of intracellular inducible NO synthase (iNOS) during differentiation led to elevated endogenous NO production. Biotin switch assays revealed a gradual increase in S-nitrosylation (SNO)-NOTCH1 and a decrease in overall NOTCH1 expression during hepatogenic differentiation. The addition of the exogenous NO donor S-nitrosoglutathione (GSNO) and the SNO inhibitor dithiothreitol (DTT) further demonstrated that the elevated expression of SNO-NOTCH1 promotes the differentiation of MSCs into mature hepatocytes. Briefly, our results fully demonstrated that the modification of the extracellular domain of NOTCH1 by NO, leading to the formation of SNO-NOTCH1, significantly promotes hepatogenic differentiation by inhibiting the Notch signalling pathway. Our study highlights the critical role of SNO-NOTCH1 in regulating the Notch signalling pathway and offers new insights into the mechanisms driving this differentiation process.
Keywords: NO; S‐nitrosylation; differentiation mechanisms; hepatogenic differentiation; mesenchymal stem cells; signalling pathways.
© 2024 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Activation of Notch1 signalling promotes multi-lineage differentiation of c-Kit(POS)/NKX2.5(POS) bone marrow stem cells: implication in stem cell translational medicine.Stem Cell Res Ther. 2015 May 9;6(1):91. doi: 10.1186/s13287-015-0085-2. Stem Cell Res Ther. 2015. PMID: 25956503 Free PMC article.
-
Oxidative stress-induced Notch1 signaling promotes cardiogenic gene expression in mesenchymal stem cells.Stem Cell Res Ther. 2013 Apr 18;4(2):43. doi: 10.1186/scrt190. Stem Cell Res Ther. 2013. PMID: 23597145 Free PMC article.
-
17β-estradiol inhibits Notch1 activation in murine macrophage cell line RAW 264.7.Mol Biol Rep. 2024 Nov 8;51(1):1134. doi: 10.1007/s11033-024-10058-x. Mol Biol Rep. 2024. PMID: 39514048
-
Enhanced angiogenesis promoted by human umbilical mesenchymal stem cell transplantation in stroked mouse is Notch1 signaling associated.Neuroscience. 2015 Apr 2;290:288-99. doi: 10.1016/j.neuroscience.2015.01.038. Epub 2015 Jan 28. Neuroscience. 2015. PMID: 25637797
-
Signalling pathways involved in the process of mesenchymal stem cells differentiating into hepatocytes.Cell Prolif. 2015 Apr;48(2):157-65. doi: 10.1111/cpr.12165. Epub 2015 Feb 6. Cell Prolif. 2015. PMID: 25656979 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- 2024SSY06291/Foundation of Jiangxi Provincal Key Laboratory of Tissue Engineering
- QD076/First Affiliated Hospital of Gannan Medical University, Doctor Start-up Fund
- 32060232/National Natural Science Foundation of China
- SC-BiFR-001/Stem Cell Clinical Research Bi-Filing Project of First Affiliated Hospital of Gannan Medical University
- 20212BAB206075/Natural Science Foundation of Jiangxi Province
LinkOut - more resources
Full Text Sources

