APOE ɛ4 and Insulin Resistance Influence Path-Integration-Based Navigation through Distinct Large-Scale Network Mechanisms
- PMID: 39656485
- PMCID: PMC12339104
- DOI: 10.14336/AD.2024.0975
APOE ɛ4 and Insulin Resistance Influence Path-Integration-Based Navigation through Distinct Large-Scale Network Mechanisms
Abstract
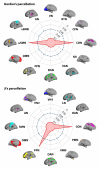
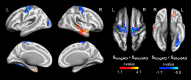
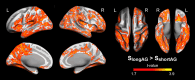
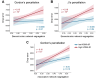
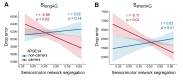
Path integration (PI), which supports navigation without external spatial cues, is facilitated by grid cells in the entorhinal cortex. These cells are often impaired in individuals at risk for Alzheimer's disease (AD). However, other brain systems can compensate for this impairment, especially when spatial cues are available. From a graph-theoretical perspective, this compensatory mechanism might manifest through changes in network segregation, indicating shifts in distinct functional roles among specialized brain regions. This study explored whether similar compensatory mechanisms are active in APOE ε4 carriers and individuals with elevated insulin resistance, both susceptible to entorhinal cortex dysfunction. We applied a graph-theoretical segregation index to resting-state fMRI data from two cohorts (aged 50-75) to assess PI performance across virtual environments. Although insulin resistance did not directly impair PI performance, individuals with higher insulin resistance demonstrated better PI with less segregated brain networks, regardless of spatial cue availability. In contrast, the APOE effect was cue-dependent: ε4 heterozygotes outperformed ε3 homozygotes in the presence of local landmarks, linked to increased sensorimotor network segregation. When spatial cues were absent, ε4 carriers exhibited reduced PI performance due to lower segregation in the secondary visual network. Controlling cortical thickness and intracortical myelin variability mitigated these APOE effects on PI, with no similar adjustment made for insulin resistance. Our findings suggest that ε4 carriers depend on cortical integrity and spatial landmarks for successful navigation, while insulin-resistant individuals may rely on less efficient neural mechanisms for processing PI. These results highlight the importance of targeting insulin resistance to prevent cognitive decline, particularly in aging navigation and spatial cognition.
Conflict of interest statement
The authors have no conflict of interest.
Figures



Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Associations of Sex, Race, and Apolipoprotein E Alleles With Multiple Domains of Cognition Among Older Adults.JAMA Neurol. 2023 Sep 1;80(9):929-939. doi: 10.1001/jamaneurol.2023.2169. JAMA Neurol. 2023. PMID: 37459083 Free PMC article.
-
Predicting cognitive decline: Deep-learning reveals subtle brain changes in pre-MCI stage.J Prev Alzheimers Dis. 2025 May;12(5):100079. doi: 10.1016/j.tjpad.2025.100079. Epub 2025 Feb 6. J Prev Alzheimers Dis. 2025. PMID: 39920001 Free PMC article.
-
Falls prevention interventions for community-dwelling older adults: systematic review and meta-analysis of benefits, harms, and patient values and preferences.Syst Rev. 2024 Nov 26;13(1):289. doi: 10.1186/s13643-024-02681-3. Syst Rev. 2024. PMID: 39593159 Free PMC article.
-
Plasma and cerebrospinal fluid amyloid beta for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2014 Jun 10;2014(6):CD008782. doi: 10.1002/14651858.CD008782.pub4. Cochrane Database Syst Rev. 2014. PMID: 24913723 Free PMC article.
References
-
- Segen V, Ying J, Morgan E, Brandon M, Wolbers T (2022). Path integration in normal aging and Alzheimer's disease. Trends Cogn Sci, 26:142-158. - PubMed
-
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K (2011). Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol, 70:960-969. - PubMed
-
- Ying J, Reboreda A, Yoshida M, Brandon MP (2023). Grid cell disruption in a mouse model of early Alzheimer's disease reflects reduced integration of self-motion cues. Curr Biol, 33:2425-2437. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
