C2230, a preferential use- and state-dependent CaV2.2 channel blocker, mitigates pain behaviors across multiple pain models
- PMID: 39656529
- PMCID: PMC11827853
- DOI: 10.1172/JCI177429
C2230, a preferential use- and state-dependent CaV2.2 channel blocker, mitigates pain behaviors across multiple pain models
Abstract
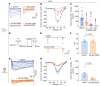
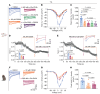
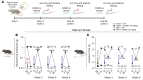
Antagonists - such as Ziconotide and Gabapentin - of the CaV2.2 (N-type) calcium channels are used clinically as analgesics for chronic pain. However, their use is limited by narrow therapeutic windows, difficult dosing routes (Ziconotide), misuse, and overdoses (Gabapentin), as well as a litany of adverse effects. Expansion of novel pain therapeutics may emerge from mechanism-based interrogation of CaV2.2. Here, we report the identification of C2230, an aryloxy-hydroxypropylamine, as a CaV2.2 blocker. C2230 trapped and stabilized inactivated CaV2.2 in a slow-recovering state and accelerated the open-state inactivation of the channel, conferring an advantageous use-dependent inhibition profile. C2230 inhibited CaV2.2 during high-frequency stimulation, while sparing other voltage-gated ion channels. C2230 inhibited CaV2.2 in dorsal root and trigeminal ganglia neurons from rats, marmosets, and humans in a G-protein-coupled-receptor-independent manner. Further, C2230 reduced evoked excitatory postsynaptic currents and excitatory neurotransmitter release in the spinal cord, leading to relief of neuropathic, orofacial, and osteoarthritic pain-like behaviors via 3 different routes of administration. C2230 also decreased fiber photometry-based calcium responses in the parabrachial nucleus, mitigated aversive behavioral responses to mechanical stimuli after neuropathic injury, and preserved protective pain responses, all without affecting motor or cardiovascular function. Finally, site-directed mutation analysis demonstrated that C2230 binds differently than other known CaV2.2 blockers, making it a promising lead compound for analgesic development.
Keywords: Calcium channels; Neuroscience; Pain; Pharmacology.
Figures










Comment in
- The N-type calcium channel rises from the ashes doi: 10.1172/JCI189308
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

