Treg cells promote decidual vascular remodeling and modulate uterine NK cells in pregnant mice
- PMID: 39656539
- PMCID: PMC11790030
- DOI: 10.1172/jci.insight.169836
Treg cells promote decidual vascular remodeling and modulate uterine NK cells in pregnant mice
Abstract
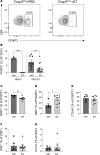
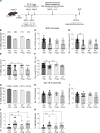
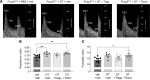
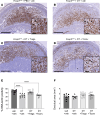
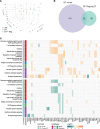
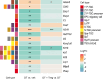
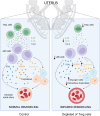
Regulatory T (Treg) cells are essential for maternal immune tolerance of the fetus and placenta. In preeclampsia, aberrant Treg cell tolerance is implicated, but how Treg cells affect the uterine vascular dysfunction thought to precede placental impairment and maternal vasculopathy is unclear. We used Foxp3-diphtheria toxin receptor mice to test the hypothesis that Treg cells are essential regulators of decidual spiral artery adaptation to pregnancy. Transient Treg cell depletion during early placental morphogenesis caused impaired remodeling of decidual spiral arteries, altered uterine artery function, and fewer Dolichos biflorus agglutinin+ uterine natural killer (uNK) cells, resulting in late-gestation fetal loss and fetal growth restriction. Replacing the Treg cells by transfer from wild-type donors mitigated the impact on uNK cells, vascular remodeling, and fetal loss. RNA sequencing of decidua revealed genes associated with NK cell function and placental extravillous trophoblasts were dysregulated after Treg cell depletion and normalized by Treg cell replacement. These data implicate Treg cells as essential upstream drivers of uterine vascular adaptation to pregnancy, through a mechanism likely involving phenotypic regulation of uNK cells and trophoblast invasion. The findings provide insight into mechanisms linking impaired adaptive immune tolerance and altered spiral artery remodeling, 2 hallmark features of preeclampsia.
Keywords: Immunology; NK cells; Obstetrics/gynecology; Reproductive biology; T cells.
Figures



References
MeSH terms
LinkOut - more resources
Full Text Sources

