The soluble guanylate cyclase activator runcaciguat significantly improves albuminuria in patients with chronic kidney disease: a randomized placebo-controlled clinical trial
- PMID: 39656628
- PMCID: PMC12123308
- DOI: 10.1093/ndt/gfae261
The soluble guanylate cyclase activator runcaciguat significantly improves albuminuria in patients with chronic kidney disease: a randomized placebo-controlled clinical trial
Abstract
Background and hypothesis: In chronic kidney disease (CKD) the nitric oxide (NO)-soluble guanylate cyclase (sGC)-cyclic guanosine monophosphate (cGMP) pathway is impaired. Runcaciguat, an sGC activator, activates heme-free sGC, restoring cGMP production. This phase 2a trial studied the efficacy, safety, and tolerability of runcaciguat in CKD patients with or without sodium-glucose co-transporter-2 inhibitor (SGLT2i).
Methods: Patients with CKD and established atherosclerotic cardiovascular disease or heart failure, plus type 2 diabetes (T2D) and/or hypertension, were enrolled. All were receiving stable maximum tolerated renin-angiotensin system inhibitors with or without SGLT2i. They were randomized 3:1 to runcaciguat once daily, titrated weekly (30-120 mg if tolerated), or placebo for 8 weeks. The primary efficacy endpoint was urine albumin-to-creatinine ratio (UACR) (average of post-randomization Days 22, 29, and 57 vs baseline). CONCORD was separately powered for CKD and T2D with stable SGLT2i comedication, CKD and T2D without SGLT2i, and non-diabetic CKD.
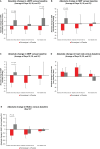
Results: Of 243 patients randomized, 229 were included in the full analysis set (FAS) and 170 in the per-protocol set (PPS). In the PPS, UACR decreased by -45.2% versus placebo with runcaciguat in patients with CKD without SGLT2i (P < 0.001) and by -48.1% versus placebo in patients with CKD taking SGLT2i (P = 0.02) In the FAS, the relative reductions were -46.9% (P < 0.001) and -44.8% (P = 0.01), respectively. No significant difference was observed between patients with or without SGLT2i. In non-diabetic CKD, UACR was reduced versus baseline with runcaciguat, but the change was not statistically significant (P = 0.10). Serious treatment-emergent adverse events were reported in 7% of patients receiving runcaciguat and 8% receiving placebo.
Conclusion: Runcaciguat improved albuminuria in patients with CKD, irrespective of concomitant SGLT2i. Runcaciguat was well tolerated. sGC activation may represent a novel kidney-protective treatment in CKD patients (funded by Bayer AG; ClinicalTrials.gov number, NCT04507061).
Keywords: albuminuria; chronic kidney disease; sodium-glucose co-transporter-2 inhibitors; soluble guanylate cyclase activator; type 2 diabetes.
© The Author(s) 2024. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
Figures




References
-
- International Society of Nephrology . ISN Global Kidney Health Atlas. 2023. Available at: https://www.theisn.org/initiatives/global-kidney-health-atlas/ (25 March 2024, date last accessed).
-
- Foreman KJ, Marquez N, Dolgert A et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet North Am Ed 2018;392:2052–90. 10.1016/S0140-6736(18)31694-5 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

