Assessment of salivary microRNA by RT-qPCR: Facing challenges in data interpretation for clinical diagnosis
- PMID: 39656703
- PMCID: PMC11630609
- DOI: 10.1371/journal.pone.0314733
Assessment of salivary microRNA by RT-qPCR: Facing challenges in data interpretation for clinical diagnosis
Abstract
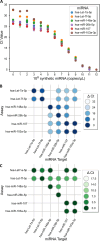
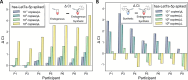
Salivary microRNAs (miRNAs) have been recently revealed as the next generation of non-invasive biomarkers for the diagnostics of diverse diseases. However, their short and highly homologous sequences make their quantification by RT-qPCR technique highly heterogeneous and study dependent, thus limiting their implementation for clinical applications. In this study, we evaluated the use of a widely used commercial RT-qPCR kit for quantification of salivary miRNAs for clinical diagnostics. Saliva from ten healthy volunteers were sampled four times within a three month time course and submitted for small RNA extraction followed by RT-qPCR analysed. Six miRNAs with different sequence homologies were analysed. Sensitivity and specificity of the tested miRNA assays were corroborated using synthetic miRNAs to evaluate the reliability of all tested assays. Significant variabilities in expression profiles of six miRNAs from ten healthy participants were revealed, yet the poor specificity of the assays offered insufficient performance to associate these differences to biological context. Indeed, as the limit of quantification (LOQ) concentrations are from 2-4 logs higher than that of the limit of detection (LOD) ones, the majority of the analysis for salivary miRNAs felt outside the quantification region. Most importantly, a remarkable number of crosstalk reactions exhibiting considerable OFF target signal intensities was detected, indicating their poor specificity and limited reliability. However, the spike-in of synthetic target miRNA increased the capacity to discriminate endogenous salivary miRNA at the LOQ concentrations from those that were significantly lower. Our results demonstrate that comparative analyses for salivary miRNA expression profiles by this commercial RT-qPCR kit are most likely associated to technical limitations rather than to biological differences. While further technological breakthroughs are still required to overcome discrepancies, standardization of rigorous sample handling and experimental design according to technical parameters of each assay plays a crucial role in reducing data inconsistencies across studies.
Copyright: © 2024 Hofstadt et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures



References
-
- Hindson J Salivary miRNA signature for ESCC. Nat Rev Gastroenterol Hepatol [Internet]. 2023. Aug 8 [cited 2023 Aug 16]; Available from: https://www.nature.com/articles/s41575-023-00837-5 doi: 10.1038/s41575-023-00837-5 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

