Cost effectiveness of empagliflozin in adult patients with chronic kidney disease in the Netherlands
- PMID: 39656735
- PMCID: PMC11630597
- DOI: 10.1371/journal.pone.0315509
Cost effectiveness of empagliflozin in adult patients with chronic kidney disease in the Netherlands
Abstract
Aim: The recent EMPA-KIDNEY trial showed evidence for preventing disease progression in adult patients with chronic kidney disease (CKD) treated with empagliflozin. It is however yet unknown if use of empagliflozin is cost effective in the Netherlands. We aimed to evaluate the cost effectiveness of empagliflozin in adult patients with CKD in the Netherlands.
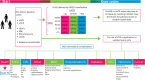
Methods: A cost-effectiveness analysis was conducted using a Markov state microsimulation model, simulating kidney progression of CKD patients with eGFR <90 ml/min per 1.73 m2 comparing empagliflozin plus standard of care (SoC) and SoC alone. KDIGO classification was used to describe the risk of CKD progression. The input data were taken from the EMPA-KIDNEY trial (baseline characteristics, treatment effect, and utilities), and published data and national sources were used for general population mortality, treatment and event costs. The analyses were performed from a societal perspective with applying a lifetime horizon. Discounting was done according to the Dutch pharmacoeconomic guidelines. The incremental cost-effectiveness ratio (ICER) was compared to a willingness-to-pay threshold of €50,000/QALY. Deterministic and probabilistic sensitivity analyses were performed to explore the impact of uncertainty around the input parameters.
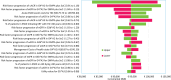
Results: The base-case results showed total discounted costs for empagliflozin plus SoC and SoC alone of €200,193 and €234,574 respectively, indicating total savings of €34,380. Empagliflozin plus SoC was associated with higher total discounted health benefits of 11.06 life years (LYs) and 9.01 quality-adjusted life years (QALYs), compared with 9.74 LYs and 7.79 QALYs for SoC alone, resulting in an additional 1.31 LYs and 1.22 QALYs for empagliflozin plus SoC. Empagliflozin plus SoC is a dominant alternative compared to SoC alone. Sensitivity analyses confirmed the robustness of the findings and conclusion.
Conclusion: Using empagliflozin in addition to SoC in adult patients with CKD is likely to be cost saving compared to the current SoC in the Netherlands, irrespective of diabetes status and albuminuria.
Copyright: © 2024 Slob et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
CB and MP receive grants and honoraria from various pharmaceutical companies, including Boehringer Ingelheim. They are both shareholders of Health-Ecore, the Netherlands. TF, BS and LJ are employed as consultants at Health-Ecore, which received a consultancy fee for the conduct of this study. MW is an employee at Boehringer Ingelheim Netherlands, the funder of this study. The commercial affiliations of the authors do not alter our adherence to PLOS ONE policies on sharing data and materials. The EMPA-KIDNEY trial was initiated, designed, and conducted by the University of Oxford in collaboration with a Steering Committee of experts and Boehringer Ingelheim. The presented analyses were initiated and conducted by Boehringer Ingelheim independently from the EMPA KIDNEY Collaborative Group. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors did not receive any personal payment related to the development of this manuscript. Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Figures

References
-
- De Grauw W, De Leest K, Schenk P, Scherpbier-De Haan N, Tjin-A-Ton J, Tuut M, et al. NHG-Standaard-Chronische nierschade. 2018;
-
- Kidney Disease Surveillance System. [cited 2023 Oct 20]. Available from: https://nccd.cdc.gov/ckd/default.aspx
-
- NICE guideline. Chronic kidney disease: assessment and management. 2021. Available from: www.nice.org.uk/guidance/ng203 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

