Increased Anion Exchanger-1 (Band 3) on the Red Blood Cell Membrane Accelerates Scavenging of Nitric Oxide Metabolites and Predisposes Hypertension Risks
- PMID: 39656872
- PMCID: PMC11815584
- DOI: 10.1093/function/zqae052
Increased Anion Exchanger-1 (Band 3) on the Red Blood Cell Membrane Accelerates Scavenging of Nitric Oxide Metabolites and Predisposes Hypertension Risks
Abstract
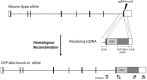
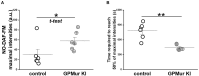
The erythrocyte membrane is highly specialized with ∼1 million anion exchanger-1 (AE1) per cell for rapid membrane permeation of HCO3-(aq), as most blood CO2(g) is carried in this hydrated anionic form. People with the GP.Mur blood type have more AE1 on their erythrocyte membrane, and they excrete CO2(g) more efficiently. Unexpectedly, GP.Mur/increased AE1 is also associated with higher blood pressure (BP). To solve this, we knocked the human GYP.Mur gene into C57BL/6J mice at 3'-UTR of GYPA to generate GPMur knock-in (KI) mice. KI of human GYP.Mur increased murine AE1 expression on the red blood cells (RBC). GPMur KI mice were naturally hypertensive, with normal kidney functions and lipid profiles. Blood NO3- [the stable nitric oxide (NO) reservoir] was significantly lower in the GPMur mice. GPMur KI also accelerated AE1-mediated NO2- influx into the RBCs and intraerythrocytic NO2-/NO processing. From tests with different categories of antihypertensives, hypertension in GPMur mice responded best to direct arterial vasodilator hydralazine, suggesting that vasodilator deficiency is the leading cause of "GPMur/AE1-triggered hypertension." In conclusion, we showed that GPMur/increased AE1 predisposed hypertension risks. Mechanistically, higher AE1 expression increased RBC membrane permeability for NO2- and consequently accelerated erythroid NO2-/NO metabolism; this is associated with lower NO bioavailability and higher BP. As hypertension affects a quarter of the world population and GP.Mur is a common Southeast Asian (SEA) blood type, this work may serve as a primer for "GPMur (biomarker)-based" therapeutic development for hypertension.
Keywords: GP.Mur (Mi.III); Miltenberger blood type; anion exchanger-1 (AE1; anion transport; band 3); blood pressure; glycophorin; hypertension; nitric oxide; nitrite; red blood cell.
© The Author(s) 2024. Published by Oxford University Press on behalf of American Physiological Society.
Conflict of interest statement
The GPMur knock-in mouse model is currently applied for patents.
Figures






References
-
- Pawloski JR, Hess DT, Stamler JS. Export by red blood cells of nitric oxide bioactivity. Nature. 2001;409(6820):622–626. - PubMed
-
- Alonso A, Arrazola A, Garciandia A, Esparza N, Gomez-Alamillo C, Diez J. Erythrocyte anion exchanger activity and intracellular pH in essential hypertension. Hypertension. 1993;22(3):348–356. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

