Race and clinical outcomes in hormone receptor-positive, HER2-negative, node-positive breast cancer in the randomized RxPONDER trial
- PMID: 39656951
- PMCID: PMC12058262
- DOI: 10.1093/jnci/djae314
Race and clinical outcomes in hormone receptor-positive, HER2-negative, node-positive breast cancer in the randomized RxPONDER trial
Abstract
Background: The phase III RxPONDER trial has affected treatment for node-positive (1-3), hormone receptor-positive, HER2-negative breast cancer with a 21-gene recurrence score (RS) less than 26. We investigated how these findings apply to different racial and ethnic groups within the trial.
Methods: The trial randomly assigned women to endocrine therapy (ET) or to chemotherapy plus ET. The primary clinical outcome was invasive disease-free survival (IDFS), with distant relapse-free survival (DRFS) as a secondary outcome. Multivariable Cox models were used to evaluate the association between race/ethnicity and survival outcomes, adjusting for clinicopathological characteristics, RS, and treatment.
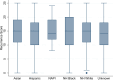
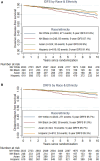
Results: A total of 4048 women with self-reported race/ethnicity were included: Hispanic (15.1%), non-Hispanic Black (NHB) (6.1%), Native American/Pacific Islander (0.8%), Asian (8.0%), and non-Hispanic White (NHW) (70%). No differences in RS distribution, tumor size, or number of positive nodes were observed by race/ethnicity. Relative to NHWs, IDFS was worse for NHB participants (5-year IDFS 91.6% vs 87.1%, HR = 1.37; 95% CI = 1.03 to 1.81) and better for Asians (91.6% vs 93.9%, HR = 0.64; 95% CI = 0.46 to 0.91). Relative to NHW, DRFS was worse for NHB participants (5-year DRFS 95.8% vs 91.0%, HR = 1.65; 95% CI = 1.17 to 2.32) and better for Asians (95.8% vs 96.7%, HR = 0.59; 95% CI = 0.37 to 0.95). Adjusting for clinical characteristics, particularly body mass index, diminished the effect of race on outcomes. Chemotherapy treatment efficacy did not differ by race/ethnicity.
Conclusions: NHB women had worse clinical outcomes compared with NHWs in the RxPONDER trial despite similar RS and comparable treatment. Our study emphasizes the persistent racial disparities in breast cancer outcomes while highlighting complex interactions among contributing factors.
Trial registration: ClinicalTrials.gov: NCT01272037.
© The Author(s) 2024. Published by Oxford University Press. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
Dr Abdou reported consulting fees from Exact Sciences and AstraZeneca outside the submitted work. Dr Meric-Bernstam reported consulting fees from AbbVie, AstraZeneca Pharmaceuticals, Becton Dickinson, Calibr (a division of Scripps Research), Daiichi Sankyo, EcoR1 Capital, eFFECTOR Therapeutics, Exelixis, GT Aperion, t Incyte, Infinity Pharmaceuticals, Jazz Pharmaceuticals, LegoChem Biosciences, Lengo Therapeutics, Menarini Group, Molecular Templates, Protai Bio, Roche, Tallac Therapeutics, Zymeworks, advisory committee for Black Diamond Therapeutics, Biovica, Eisai Medical Research, FogPharma, Harbinger Health, Karyopharm Therapeutics, LOXO-Oncology Mersana Therapeutics, OnCusp Therapeutics, Sanofi Pharmaceuticals, Seagen, Theratechnologies, Zentalis Pharmaceuticals, sponsored research to the institution from Jazz Pharmaceuticals, Zymeworks, Aileron Therapeutics, Inc, AstraZeneca, Bayer Healthcare Pharmaceutical, Calithera Biosciences Inc, Curis Inc, CytomX Therapeutics Inc, Daiichi Sankyo Co. Ltd, Debiopharm International, eFFECTOR Therapeutics, Genentech Inc, Guardant Health Inc, Klus Pharma, Takeda Pharmaceutical, Novartis, Puma Biotechnology Inc, Taiho Pharmaceutical Co, honoraria from Dava Oncology, and travel support from European Organisation for Research and Treatment of Cancer (EORTC), European Society for Medical Oncology (ESMO), Cholangiocarcinoma Foundation, Dava Oncology, all outside the submitted work. Dr Albain reports advisory/consulting fees from Exact Sciences (previously Genomic Health, Inc), SeaGen, Agendia, and research funding from QuantumLeap Healthcare, all outside the submitted work. Dr Hayes reported consulting fees from Artera.ai, Arvenis, BioVeca, BioTheranostics an Hologic Company, Cellworks, Centrix, Cepheid, Delphi Diagnostics, EPIC Sciences, EXACT Sciences, Freenome, Guardant, Macrogenics, Oncocyte, Stratipath, Tempus, Turnstone Biologics, and Xilis, research support to the institution from Astra Zeneca, Menarini Silicon Biosystems, Pfizer, Cepheid, and Angle, stock options from InBiomotion, Xilis, and CellWorks, honoraria from UpToDate and CancerExpertNow, and the University of Michigan holds a patent for which Dr Hayes is the named investigator and which was licensed to Menarini Silicon Biosystems from whom Dr Hayes received annual royalties ending January 1, 2022, all outside the submitted work. Dr Lin reported research Institutional funding from Genentech, Pfizer, Merck, Seattle Genetics, Zion Pharmaceuticals, Olema Pharmaceuticals, and AstraZeneca, consulting fees from Seattle Genetics, Daichii-Sankyo, AstraZeneca, Olema Pharmaceuticals, Janssen, Blueprint Medicines, Stemline/Menarini, Artera Inc, and Eisai, and travel support (outside consulting fees) from Olema Pharmaceuticals, all outside the submitted work. Dr Chia reported advisory fees from Novartis, Hoffmann LaRoche, Pfizer, Eli Lilly, AstraZeneca, Gilead, Merck, and Daiichi Sankyo, all outside the submitted work. Dr Dhesy-Thind reported consulting fees from Novartis Canada Pharmaceuticals Inc; Seagen and honoraria from Pfizer Canada; Novartis Canada Pharmaceuticals Inc, outside the submitted work. Dr Delaloge reported institutional grants from Pfizer, Novartis, AstraZeneca, Roche, Lilly, Orion, Amgen, Sanofi, BMS, Pierre Fabre, Taiho, European Commission, and French Government, travel support from Novartis, Roche, and Seagen, and advisory fees to the institution from BIG international group, Pfizer, Gilead, Sanofi, Elsan, Besins, Decibio, AstraZeneca, all outside the submitted work. Dr Shak reported consulting fees from Exact Sciences, N-Power Medicine, and Genomic Life, all outside submitted work. Dr Sharma reported research funding from Merck, Novartis, Gilead, and Bristol Myers Squibb, royalties from UpToDate, and consulting fees from Merck, Gilead, Genzyme Corporation (Sanofi), Novartis, AstraZeneca, GSK, Pfizer, and Exact Sciences, all outside the submitted work. Dr Unger reported consulting fees from AstraZeneca, outside the submitted work. Dr Tripathy reported consulting fees from Novartis, Pfizer, GlaxoSmithKline, Genomic Heath, AstraZeneca, Oncopep, Sermonix, Personalis, Ambrx, Gilead, Roche, Menarini, and Jazz Pharmaceuticals, institutional research support from Novartis, Polyphor, Pfizer, and Ambrx, and travel support from Novartis and AstraZeneca, all outside the submitted work. Dr Pusztai reported consulting fees and honoraria for advisory board participation from Pfizer, Astra Zeneca, Merck, Novartis, Bristol-Myers Squibb, Stemline-Menarini, GlaxoSmithKline, Genentech/Roche, Personalis, Daiichi, Natera, Agendia, and Exact Sciences and institutional research funding from Menarini, AstraZeneca, Merck, Pfizer, and Bristol Myers Squibb, all outside the submitted work. Dr Kalinsky reported the following: Spouse, Stock: EQRX (Prior Employee), ADC Therapeutics, consulting fees from Lilly, Novartis, Genentech/Roche, Gilead, Seattle Genetics, Astra Zeneca, Daiichi Sankyo, Puma Biotechnology, Mersana, Menarini Silicon Biosystems, Myovant Sciences, Takeda, Prelude Therapeutics, eFFECTOR Therapeutis, Regor, RayzeBio, Relay Therapuetics, Merck, and Institutional funding from Novartis, Genentech/Roche, Lilly, Seattle Genetics, Astra Zeneca, Daichi Sankyo, Ascentage, all outside the submitted work. No other disclosures were reported.
Figures

References
-
- Office of the Commissioner. Collection of Race and Ethnicity Data in Clinical Trials. Updated Thu, 08/10/2023—16:04.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

