Health care systems as determinants of outcomes in multiple myeloma: final results from the Latin American MYLACRE study
- PMID: 39657126
- PMCID: PMC11950971
- DOI: 10.1182/bloodadvances.2024013838
Health care systems as determinants of outcomes in multiple myeloma: final results from the Latin American MYLACRE study
Abstract
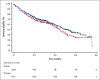
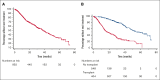
Although systemic therapy for multiple myeloma (MM) has evolved considerably over the past 2 decades, state-of-the-art treatment is not uniformly available in Latin America. In some countries, disparities between the public and private sectors in clinical presentation, access to novel agents, and transplantation are striking, with the public sector lagging. We conducted a multicenter, observational study of patients with MM in 5 Latin American countries (Argentina, Brazil, Colombia, Mexico, and Panama). We enrolled patients aged ≥18 years diagnosed with MM between January 2016 and June 2021, using data collected between May 2019 and June 2022. We categorized institutions as "public" when primarily funded by federal or local government, and "private" when financed mostly or completely by other sources. We analyzed 1029 patients, 1021 of whom could be classified into public (n = 339) and private (n = 682) institutions. These 2 groups differed in many respects, with patients from the latter having better baseline prognostic features (including eligibility to transplantation) and receiving combinations of immunomodulatory drugs and proteasome inhibitors, as well as anti-CD38 antibodies, more frequently than patients from public institutions. Among 960 patients with complete data for this analysis, the median overall survival was 44.6 months in public institutions and 53.3 months in private institutions (hazard ratio, 0.84; 95% confidence interval, 0.67-1.04; P = .109). Our results indicate diagnostic and therapeutic shortcomings in the management of MM in Latin America, with important gaps in patient profile, treatment patterns and long-term outcomes between public and private institutions. This trial was registered at www.clinicaltrials.gov as #NCT03955900.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: V.H. reports study funding from Janssen; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AbbVie, Amgen, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Pfizer, Sanofi, and Takeda; support for attending meetings and/or travel from Janssen; and participation on a data safety monitoring board or advisory board for Janssen. R.G. reports study funding from Janssen; honoraria for lectures from Janssen; support for attending national and international meetings, including travel expenses, from Janssen; and support for attending meetings and/or travel from Takeda and AbbVie. K.G. reports study funding from Janssen. G.R. reports study funding from Janssen; consulting fees from Janssen, Sanofi, and Pfizer; payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Janssen and Sanofi; support for attending meetings and/or travel from Janssen and Sanofi; and participation on advisory board for Janssen, Pfizer, and Sanofi. N.S. reports study funding from Janssen; staff training in multiple myeloma from Janssen; payment or honoraria for lectures from Janssen and Sanofi; travel grant from Sanofi and Takeda; and participation on advisory boards for Janssen, Sanofi, and Pfizer. R.B. received study funding from Janssen. A.M. received study funding from Janssen; reports grants or contracts from any entity from CNPq and FAPERJ; reports consulting fees from Janssen, Takeda, Pfizer, Novartis, Sanofi, Amgen, AbbVie, and Bristol Myers Squibb; reports payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Janssen, Takeda, Pfizer, Sanofi, Amgen, and Bristol Myers Squibb; reports participation on a data safety monitoring boards or advisory boards for Janssen, Bristol Myers Squibb, and Pfizer; and reports leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid, from Associação Brasileira de Hematologia, Hemoterapia e Terapia Celular. G.Q. received study funding from Janssen; reports payment or honoraria for lectures from AstraZeneca and Amgen; received support for attending meetings and/or travel from Janssen and Roche; and reports participation on advisory boards for Janssen, Sanofi, and AstraZeneca. M.S.C. received study funding from Janssen; reports grants or contracts from any entity from Bristol Myers Squibb; received payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from AbbVie, AstraZeneca, Raffo, and Takeda; received support for attending meetings and/or travel from Takeda, AbbVie, Raffo, Sanofi, and BeiGene; reports participation on a data safety monitoring boards or advisory boards for Raffo and Sanofi; and reports leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid, and membership of the directive board (treasurer) with Argentinian Society of Hematology (unpaid). W.M.T.B. reports study funding from Janssen; received payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Janssen, Bristol, and Sanofi; and reports support for attending meetings and/or travel from Janssen and Sanofi. C.C.V. reports study funding from Janssen. E.C. reports study funding from Janssen; research funding from Janssen; and payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Janssen, Amgen, Takeda, Bristol Myers Squibb, GlaxoSmithKline, and Alexion. A.I.E. and G.C. report study funding from Janssen. J.B. reports study funding from Janssen. F.L.M. reports study funding from Janssen; is a researcher in clinical trials sponsored by Janssen-Cilag, Takeda, Novartis, and Bristol Myers Squibb; reports academical scientific presentations or products from lecturer in events sponsored by Janssen-Cilag, Takeda, Bristol Myers Squibb, and Amgen; reports consultancy activities for advisory boards sponsored by Janssen-Cilag, Takeda, and Bristol Myers Squibb; reports support for participation in congresses by Takeda, Janssen-Cilag, Bristol Myers Squibb, and Sanofi; reports consulting fees from Janssen-Cilag, Takeda, Bristol Myers Squibb, Amgen, Sanofi, and Pfizer; reports payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Janssen-Cilag, Takeda, Bristol Myers Squibb, Amgen, Takeda, Sanofi, and Pfizer; reports payment for expert testimony from Janssen-Cilag, Takeda, Bristol Myers Squibb, Amgen, Takeda, Sanofi, and Pfizer; reports support for attending meetings and/or travel from Janssen-Cilag, Takeda, Bristol Myers Squibb, Amgen, Takeda, Sanofi, and Pfizer; and reports participation on a data safety monitoring boards or advisory boards for Janssen-Cilag, Takeda, Bristol Myers Squibb, Amgen, Takeda, Sanofi, and Pfizer. J.F. reports study funding from Janssen; payment or honoraria for lectures from Janssen, Sanofi, and AbbVie; support for attending meetings from Amgen, AbbVie, and Janssen; and participation on advisory boards for Sanofi and Valentech Pharma. C.L.S.M. reports study funding from Janssen; received grants from Amgen; reports payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Pfizer, Amgen, AbbVie, Astellas, Janssen, Takeda, and Novo Nordisk; reports support for attending meetings and/or travel from Amgen, Novartis, and Janssen; reports participation on a data safety monitoring boards or advisory boards for Janssen and Sanofi; and reports leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid, as/for Vice-presidente Association Colombiana de Hemato-Oncología and Vocal del Consejo Colombiano de acreditación y recertificación medica. M.L. reports study funding from Janssen; reports grants or contracts from any entity from Roche, Bayer, AstraZeneca, and Janssen; received consulting fees from Roche, AstraZeneca, and Janssen; received payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Roche, Astellas, and Janssen; received payment for expert testimony from Eli Lily, Roche, and Bristol; and received support for attending meetings and/or travel from Amgen, Janssen, Roche, AstraZeneca, and Pfizer. H.P. reports study funding from Janssen; holds stock or stock options in Janssen; and is an employee of, and owns stocks in, Johnson & Johnson. M.F. reports study funding from Janssen and is an employee of Janssen. J.S. reports study funding from, and is an employee of Janssen. D.C.T. reports study funding from, and is an employee of, Janssen, and holds stock or stock options in Johnson & Johnson.
Figures

References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. - PubMed
-
- van de Donk N, Pawlyn C, Yong KL. Multiple myeloma. Lancet. 2021;397(10272):410–427. - PubMed
-
- World Health Organization Cancer fact sheets. https://gco.iarc.fr/today/en/fact-sheets-cancers
-
- World Health Organization Multiple Myeloma. https://gco.iarc.who.int/media/globocan/factsheets/cancers/35-multiple-m...
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

