Lisocabtagene maraleucel for relapsed/refractory large B-cell lymphoma: a cell therapy consortium real-world analysis
- PMID: 39657136
- PMCID: PMC11993828
- DOI: 10.1182/bloodadvances.2024014164
Lisocabtagene maraleucel for relapsed/refractory large B-cell lymphoma: a cell therapy consortium real-world analysis
Abstract
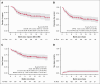
Lisocabtagene maraleucel (liso-cel) is an autologous CD19-directed chimeric antigen receptor T-cell therapy approved for the treatment of relapsed/refractory large B-cell lymphoma. We present a multicenter retrospective study evaluating safety, efficacy, and resource use of liso-cel in the standard-of-care setting. Patients received commercial liso-cel at 7 US medical centers, and patient selection, toxicity management, and disease assessment followed institutional practices. Among 101 patients who received infusion, the median age was 71 years (35% aged ≥75 years), 68% had a Charlson comorbidity index score of ≥3, and 10% had secondary central nervous system involvement. Median number of prior therapies was 3; and because of comorbidities, 33% would have been ineligible for the TRANSCEND study. Bridging therapy was used in 60% (43% received polatuzumab-based treatment). Any-grade cytokine-release syndrome occurred in 49% (3% grade ≥3) with any-grade immune effector cell-associated neurotoxicity syndrome occurring in 26% (10% grade ≥3). The overall response rate (ORR) to bridging therapy was 45%, with 18% achieving a complete response (CR). Following liso-cel infusion, the day 90 ORR was 66% (60% CR); and with a median follow-up of 15.5 months, 12-month progression-free survival (PFS) and overall survival (OS) were 55% and 68%, respectively. A normal lactate dehydrogenase level before lymphodepletion was associated with improved PFS and OS. These analyses confirm similar efficacy and safety of commercial liso-cel compared with pivotal trial results. Notably, these outcomes were achieved in patients predominantly of advanced age and with significant comorbidities. Results also likely reflect advancements in patient selection, toxicity management, and the use of novel bridging strategies.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: P.A.R. is a consultant and/or advisory board member for AbbVie, Novartis, Bristol Myers Squibb, ADC Therapeutics, Kite/Gilead, Sana Biotechnology, Nektar Therapeutics, Intellia Therapeutics, CVS Caremark, Genmab, BeiGene, Janssen, Pharmacyclics, and Genentech/Roche; reports honoraria from Adaptive Biotechnologies; and reports research support from Bristol Myers Squibb, Kite Pharma, Novartis, CRISPR Therapeutics, Calibr, Xencor, Fate Therapeutics, AstraZeneca, Genentech/Roche, Cellectis, Cargo Therapeutics, and Tessa Therapeutics. L.J.N. has served as a consultant and/or advisory board member for AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb, Caribou Biosciences, Daiichi Sankyo, Genentech/Roche, Genmab, Gilead/Kite, Janssen, Incyte, Ipsen, Merck, Novartis, Regeneron, and Takeda, and reports research support from Bristol Myers Squibb, BeiGene, Caribou Biosciences, Daiichi Sankyo, Genentech/Roche, Genmab, Gilead/Kite, IGM Biosciences, Ipsen, Janssen, Merck, Novartis, and Takeda. A.L. has received research funding from Gilead. N.A. reports serving on advisory boards for Bristol Myers Squibb and Legend Biotech; received consultancy fees from Kite/Gilead; and reports institutional research funding from Kite/Gilead. R.T.M. reports serving as a consultant for Autolus, Kite/Gilead, and Novartis; receiving research support from Gamida, Kite/Gilead, OrcaBio, and Novartis; serving on a scientific steering committee for Artiva; and participating in data and safety monitoring boards for Athersys, Novartis, Century Therapeutics, and VorPharma and a patent with Athersys. S.J.S. reports consultancy for Novartis, Regeneron, Nordic, MorphoSys, MustangBio, Incyte, Genentech/Roche, Janssen, Legend Biotech, Loxo, Acerta, BeiGene, Celgene, Nanovector, and Pharmacyclics, and research funding from Novartis, Incyte, Genentech/Roche, Celgene, Merck, DTRM, Juno Therapeutics, AbbVie, Adaptive Biotechnologies, and TG Therapeutics. A.I.C. has served as a consultant for Elsevier and ADC Therapeutics, and reports research support from Fate Therapeutics, Novartis, and Kite. O.O.O. serves as a consultant and/or advisory board member for Pfizer, Kite, Gilead, AbbVie, Janssen, TGR Therapeutics, ADC Therapeutics, Novartis, Epizyme, Nektar, Cargo, Caribou, Century, Autolus, Bioheng, Allogene; reports honoraria from Gilead and ADC Therapeutics; and reports institutional funding from Kite, Pfizer, Daiichi Sankyo, Cargo, Caribou, Sana, Century, and Allogene. V.B. serves on an advisory board for CRISPR, ADC Therapeutics, AstraZeneca, Allogene, and BeiGene; serves as a data and safety monitoring board member for Miltenyi; and reports funding from Incyte, Citius, and Gamida Cell. J.P.M. reports honoraria from Kite, AlloVir, Bristol Myers Squibb, Novartis, CRISPR, Nektar Therapeutics, Caribou Bio, Sana Biotechnologies, Legend Biotech, and Cargo Therapeutics. M.-A.P. reports honoraria from Adicet, Allogene, AlloVir, Caribou Biosciences, Celgene, Bristol Myers Squibb, Equillium, Exevir, ImmPACT Bio, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, OrcaBio, Sanofi, Syncopation, VectivBio AG, and Vor Biopharma; serves on data and safety monitoring boards for Cidara Therapeutics and Sellas Life Sciences; serves on the scientific advisory board of NexImmune; reports ownership interests in NexImmune, Omeros, and OrcaBio; and has received institutional research support for clinical trials from Allogene, Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis. M.R.B. reports being a consultant or advisory board member with Kite/Gilead, Novartis, CRISPR Therapeutics, Autolus Therapeutics, Bristol Myers Squibb/Juno Therapeutics, Chimeric Therapeutics, IN8bio, Galapagos, Incyte, Sana Biotechnology, and Iovance Biotherapeutics, and serves on speakers' bureaus for AstraZeneca, Bristol Myers Squibb, Kite/Gilead, Servier, Sanofi, Incyte, Genmab, and ADC Therapeutics. D.L.P. serves as a consultant or advisory board member for Novartis, Kite/Gilead, Janssen (Johnson & Johnson), Bristol Myers Squibb, Angiocrine, Mirror Biologics, Capstan Therapeutics, Sana Biotechnology, and Verismo Therapeutics; reports stock and other ownership interests in Genentech/Roche (spouse former employment); reports research funding from Novartis and Bristol Myers Squibb; and reports patents, royalties, and other intellectual property with Novartis and Tmunity. The remaining authors declare no competing financial interests.
Figures
Comment in
-
CAR T-cell reality for R/R LBCL: challenges and challengers.Blood Adv. 2025 Mar 11;9(5):1230-1231. doi: 10.1182/bloodadvances.2024015525. Blood Adv. 2025. PMID: 40067335 Free PMC article. No abstract available.
References
-
- Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood. 2015;125(1):22–32. - PubMed
-
- Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010;116(12):2040–2045. - PMC - PubMed
-
- Van Den Neste E, Schmitz N, Mounier N, et al. Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL study. Bone Marrow Transplant. 2016;51(1):51–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

