Early Restrictive vs Liberal Oxygen for Trauma Patients: The TRAUMOX2 Randomized Clinical Trial
- PMID: 39657224
- PMCID: PMC11815523
- DOI: 10.1001/jama.2024.25786
Early Restrictive vs Liberal Oxygen for Trauma Patients: The TRAUMOX2 Randomized Clinical Trial
Abstract
Importance: Early administration of supplemental oxygen for all severely injured trauma patients is recommended, but liberal oxygen treatment has been associated with increased risk of death and respiratory complications.
Objective: To determine whether an early 8-hour restrictive oxygen strategy compared with a liberal oxygen strategy in adult trauma patients would reduce death and/or major respiratory complications.
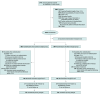
Design, setting, and participants: This randomized controlled trial enrolled adult trauma patients transferred directly to hospitals, triggering a full trauma team activation with an anticipated hospital stay of a minimum of 24 hours from December 7, 2021, to September 12, 2023. This multicenter trial was conducted at 15 prehospital bases and 5 major trauma centers in Denmark, the Netherlands, and Switzerland. The 30-day follow-up period ended on October 12, 2023. The primary outcome was assessed by medical specialists in anesthesia and intensive care medicine blinded to the randomization.
Interventions: In the prehospital setting or on trauma center admission, patients were randomly assigned 1:1 to a restrictive oxygen strategy (arterial oxygen saturation target of 94%) (n = 733) or liberal oxygen strategy (12-15 L of oxygen per minute or fraction of inspired oxygen of 0.6-1.0) (n = 724) for 8 hours.
Main outcomes and measures: The primary outcome was a composite of death and/or major respiratory complications within 30 days. The 2 key secondary outcomes, death and major respiratory complications within 30 days, were assessed individually.
Results: Among 1979 randomized patients, 1508 completed the trial (median [IQR] age, 50 [31-65] years; 73% male; and median Injury Severity Score was 14 [9-22]). Death and/or major respiratory complications within 30 days occurred in 118 of 733 patients (16.1%) in the restrictive oxygen group and 121 of 724 patients (16.7%) in the liberal oxygen group (odds ratio, 1.01 [95% CI, 0.75 to 1.37]; P = .94; absolute difference, 0.56 percentage points [95% CI, -2.70 to 3.82]). No significant differences were found between groups for each component of the composite outcome. Adverse and serious adverse events were similar across groups, with the exception of atelectasis, which was less common in the restrictive oxygen group compared with the liberal oxygen group (27.6% vs 34.7%, respectively).
Conclusions and relevance: In adult trauma patients, an early restrictive oxygen strategy compared with a liberal oxygen strategy initiated in the prehospital setting or on trauma center admission for 8 hours did not significantly reduce death and/or major respiratory complications within 30 days.
Trial registration: ClinicalTrials.gov Identifier: NCT05146700.
Conflict of interest statement
Figures



Comment in
-
Early restrictive versus liberal oxygen for trauma patients: does it make a difference?Ann Transl Med. 2025 Apr 30;13(2):12. doi: 10.21037/atm-25-33. Epub 2025 Apr 29. Ann Transl Med. 2025. PMID: 40438516 Free PMC article. No abstract available.
References
-
- American College of Surgeons . ATLS Student Course Manual, 10th Edition. 2018.
-
- Abbafati C, Abbas KM, Abbasi M, et al. ; GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204-1222. doi:10.1016/S0140-6736(20)30925-9 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

