Post-treatment duration of positivity for standard and ultra-sensitive Plasmodium falciparum antigen-based rapid diagnostic tests, a cohort study from a low-endemic setting in Namibia
- PMID: 39657364
- PMCID: PMC11683224
- DOI: 10.1016/j.ebiom.2024.105489
Post-treatment duration of positivity for standard and ultra-sensitive Plasmodium falciparum antigen-based rapid diagnostic tests, a cohort study from a low-endemic setting in Namibia
Abstract
Background: The standard malaria rapid diagnostic test (RDT) and newer ultra-sensitive RDT (uRDT) target Plasmodium falciparum histidine rich protein-2 (HRP2), which persists post-treatment. The duration of test positivity has not previously been studied in a low transmission setting.
Methods: We conducted a longitudinal cohort study in a low transmission setting in Namibia. RDT-positive individuals identified through passive and active case detection were treated and followed weekly for testing by RDT and uRDT, HRP2 quantification, quantitative PCR (qPCR) of parasitemia, and quantitative reverse transcriptase PCR (RT-PCR) of gametocytemia, until RDT and uRDT were negative for two consecutive weeks. Determinants of persistent positivity were identified using Cox proportional hazards models.
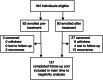
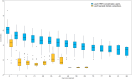
Findings: Among 137 participants with complete follow-up and no evidence of resurgence during follow-up, median duration of positivity was 42 days (range: 3-98 range) for RDT, compared to 67 days (range 12-105) for uRDT. In a sub-analysis of those with laboratory data before treatment (n = 60), drug resistance did not explain persistent positivity. Younger age (<15 years versus ≥15 years: aHR: 1.85, 95% CI 1.04-3.30, and 1.67, 95% CI 0.96-2.89, for RDT and uRDT, respectively), higher initial parasite density (highest versus lowest tertile: aHR 0.11, 95% CI 0.04-0.32 and 0.19, 95% CI 0.07-0.48 for RDT and uRDT, respectively), and persistent parasitemia (≥7 days versus reference of <7 days, aHR 0.39, 95% CI 0.20-0.76, and 0.40, 95% CI 0.21-0.76 for RDT and uRDT, respectively) were associated with longer duration of positivity.
Interpretation: Duration of RDT/uRDT positivity was more than double compared to reports from higher endemic settings, potentially due to lower population immunity to clear parasite DNA and antigen. Prolonged duration of positivity compromises their use to detect current infection, but increased detection of recent infection can facilitate surveillance and inform elimination efforts.
Funding: The project was funded by the Bill and Melinda Gates Foundation (A128488 and INV1135840), Horchow Family Fund (5300375400), and Chan Zuckerberg Biohub.
Keywords: Histidine-rich protein; Low transmission; Low-endemic; Malaria; Malaria elimination; Persistence.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests All authors declare no competing interests.
Figures


References
-
- WHO . 3rd ed. World Health Organization; Geneva, Switzerland: 2015. Guidelines for the treatment of malaria.
-
- UNITAID . 3rd ed. World Health Organization; Geneva, Switzerland: 2016. Malaria diagnostics technology and market landscape.

