Multi-modal analysis reveals tumor and immune features distinguishing EBV-positive and EBV-negative post-transplant lymphoproliferative disorders
- PMID: 39657667
- PMCID: PMC11722118
- DOI: 10.1016/j.xcrm.2024.101851
Multi-modal analysis reveals tumor and immune features distinguishing EBV-positive and EBV-negative post-transplant lymphoproliferative disorders
Abstract
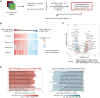
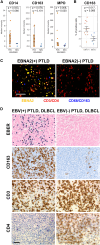
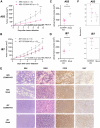
The oncogenic Epstein-Barr virus (EBV) can drive tumorigenesis with disrupted host immunity, causing malignancies including post-transplant lymphoproliferative disorders (PTLDs). PTLD can also arise in the absence of EBV, but the biological differences underlying EBV(+) and EBV(-) B cell PTLD and the associated host-EBV-tumor interactions remain poorly understood. Here, we reveal the core differences between EBV(+) and EBV(-) PTLD, characterized by increased expression of genes related to immune processes or DNA interactions, respectively, and the augmented ability of EBV(+) PTLD B cells to modulate the tumor microenvironment through elaboration of monocyte-attracting cytokines/chemokines. We create a reference resource of proteins distinguishing EBV(+) B lymphoma cells from EBV(-) B lymphoma including the immunomodulatory molecules CD300a and CD24, respectively. Moreover, we show that CD300a is essential for maximal survival of EBV(+) PTLD B lymphoma cells. Our comprehensive multi-modal analyses uncover the biological underpinnings of PTLD and offer opportunities for precision therapies.
Keywords: B cell lymphoma; CD300a; Epstein-Barr virus; multi-cohort analysis; multi-omics; post-transplant lymphoproliferative disorder; tumor microenvironment; tumor-immune interactions.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
-
- Hatton O., Strauss-Albee D.M., Zhao N.Q., Haggadone M.D., Pelpola J.S., Krams S.M., Martinez O.M., Blish C.A. NKG2A-Expressing Natural Killer Cells Dominate the Response to Autologous Lymphoblastoid Cells Infected with Epstein–Barr Virus. Front. Immunol. 2016;7:607. doi: 10.3389/fimmu.2016.00607. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

