A prognostic molecular signature of hepatic steatosis is spatially heterogeneous and dynamic in human liver
- PMID: 39657669
- PMCID: PMC11722105
- DOI: 10.1016/j.xcrm.2024.101871
A prognostic molecular signature of hepatic steatosis is spatially heterogeneous and dynamic in human liver
Abstract
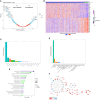
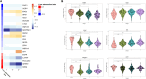
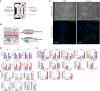
Hepatic steatosis is a central phenotype in multi-system metabolic dysfunction and is increasing in parallel with the obesity pandemic. We use a translational approach integrating clinical phenotyping and outcomes, circulating proteomics, and tissue transcriptomics to identify dynamic, functional biomarkers of hepatic steatosis. Using multi-modality imaging and broad proteomic profiling, we identify proteins implicated in the progression of hepatic steatosis that are largely encoded by genes enriched at the transcriptional level in the human liver. These transcripts are differentially expressed across areas of steatosis in spatial transcriptomics, and several are dynamic during stages of steatosis. Circulating multi-protein signatures of steatosis strongly associate with fatty liver disease and multi-system metabolic outcomes. Using a humanized "liver-on-a-chip" model, we induce hepatic steatosis, confirming cell-specific expression of prioritized targets. These results underscore the utility of this approach to identify a prognostic, functional, dynamic "liquid biopsy" of human liver, relevant to biomarker discovery and mechanistic research applications.
Keywords: diabetes; liver-on-a-chip; metabolic dysfunction-associated steatotic liver disease; non-alcoholic fatty liver disease; proteomics.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests R.S., J.E.B., and A.S.P. have filed for a patent relevant to the findings in this manuscript. R.S. is supported in part by grants from the National Institutes of Health (NIH) and the American Heart Association (AHA). R.S. has equity ownership in Thryv Therapeutics and has served as a consultant for Amgen and Cytokinetics. R.S. is a co-inventor on a patent for ex-RNA signatures of cardiac remodeling (not relevant to the current work), and other patents on proteomic signatures of fitness and lung disease. A.S.P. and E.C. are supported by the AHA Strategically Focused Research Network in Cardiometabolic Disease. J.F.K.S. and G.M. are employees of Emulate, Inc. (a maker of the liver-on-a-chip) and may hold equity interest in Emulate, Inc. S.D. holds a research grant from Bristol Myers Squibb, is a founder and holds equity in Switch Therapeutics, and is a founder and consultant and holds equity for Thryv Therapeutics. J.K. has served as a consultant to Gilead, Merck, ViiV Healthcare, and Janssen and also received research support from Gilead Sciences and Merck. R.K. is supported in part by grants from the NIH; has received grants from AstraZeneca, PneumRx/BTG, and Spiration; has received consulting fees from CVS Caremark, AstraZeneca, GlaxoSmithKline, and CSA Medical; and has received speaking fees from GlaxoSmithKline, AstraZeneca, and Boehringer Ingelheim. K.A. is supported by an AHA Career Development Award (#929347). J.A.F. serves as a consultant or advisory board member for Kynos Therapeutics, Resolution Therapeutics, Ipsen, River 2 Renal Corp., Stimuliver, Global Clinical Trial Partners, and Guidepoint and has received speaker’s fees from HistoIndex and research grant funding from GlaxoSmithKline, Intercept Pharmaceuticals, and Genentech. T.J.K. undertakes consultancy work for Perspectum, Clinnovate Health, Kynos Therapeutics, Fibrofind, HistoIndex, Concept Life Sciences, and Resolution Therapeutics and has received speaker’s fees from Incyte Corporation and Servier Laboratories. K.V.K.-J. is a member of the scientific advisory board at Dyrnamix. J.J.C. receives project funding from GE Healthcare, Siemens Healthineers, TheraTech, and the NIH. M.N. has received speaking honoraria from Cytokinetics.
Figures



Update of
-
A prognostic molecular signature of hepatic steatosis is spatially heterogeneous and dynamic in human liver.medRxiv [Preprint]. 2024 Jan 29:2024.01.26.24301828. doi: 10.1101/2024.01.26.24301828. medRxiv. 2024. Update in: Cell Rep Med. 2024 Dec 17;5(12):101871. doi: 10.1016/j.xcrm.2024.101871. PMID: 38352394 Free PMC article. Updated. Preprint.
References
-
- Riazi K., Azhari H., Charette J.H., Underwood F.E., King J.A., Afshar E.E., Swain M.G., Congly S.E., Kaplan G.G., Shaheen A.A. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet. Gastroenterol. Hepatol. 2022;7:851–861. doi: 10.1016/S2468-1253(22)00165-0. - DOI - PubMed
-
- Meyersohn N.M., Mayrhofer T., Corey K.E., Bittner D.O., Staziaki P.V., Szilveszter B., Hallett T., Lu M.T., Puchner S.B., Simon T.G., et al. Association of Hepatic Steatosis With Major Adverse Cardiovascular Events. Independent of Coronary Artery Disease. Clin Gastroenterol Hepatol. 2021;19:1480–1488.e1414. doi: 10.1016/j.cgh.2020.07.030. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

