Ceftolozane/tazobactam disrupts Pseudomonas aeruginosa biofilms under static and dynamic conditions
- PMID: 39657684
- PMCID: PMC11787898
- DOI: 10.1093/jac/dkae413
Ceftolozane/tazobactam disrupts Pseudomonas aeruginosa biofilms under static and dynamic conditions
Abstract
Background: Pseudomonas aeruginosa biofilms limit the efficacy of currently available antibacterial therapies and pose significant clinical challenges. Pseudomonal biofilms are complicated further when other markers of persistence such as mucoid and hypermutable phenotypes are present. There is currently a paucity of data regarding the activity of the newer β-lactam/β-lactamase inhibitor combination ceftolozane/tazobactam against P. aeruginosa biofilms.
Methods: We evaluated the efficacy of ceftolozane/tazobactam against clinical P. aeruginosa isolates, the laboratory isolate PAO1 and its isogenic mutS-deficient hypermutator derivative (PAOMS) grown under static and dynamic biofilm conditions. The clinical isolate collection included strains with mucoid and hypermutable phenotypes.
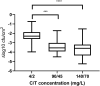
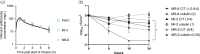
Results: Ceftolozane/tazobactam exposure led to a bactericidal (≥3 log cfu/cm2) biofilm reduction in 15/18 (83%) clinical isolates grown under static conditions, irrespective of carbapenem susceptibility or mucoid phenotype, with greater activity compared with colistin (P < 0.05). Dynamically grown biofilms were less susceptible to ceftolozane/tazobactam with active biofilm reduction (≥1 log cfu/cm2) observed in 2/3 isolates. Hypermutability did not affect the antibiofilm efficacy of ceftolozane/tazobactam in either static or dynamic conditions when comparing PAO1 and PAOMS. Consistent with the activity of ceftolozane/tazobactam as a potent inhibitor of PBP3, dramatic impacts on P. aeruginosa morphology were observed.
Conclusions: Our data demonstrate that ceftolozane/tazobactam has encouraging properties in the treatment of P. aeruginosa biofilm infections, and its activity is not diminished against mucoid or hypermutable variants at the timepoints examined.
© The Author(s) 2024. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

