Optimizing rare disorder trials: a phase 1a/1b randomized study of KL1333 in adults with mitochondrial disease
- PMID: 39657714
- PMCID: PMC11706290
- DOI: 10.1093/brain/awae308
Optimizing rare disorder trials: a phase 1a/1b randomized study of KL1333 in adults with mitochondrial disease
Abstract
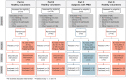
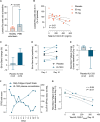
Over the past two decades there has been increased interest in orphan drug development for rare diseases. However, hurdles to clinical trial design for these disorders remain. This phase 1a/1b study addressed several challenges, while evaluating the safety and tolerability of the novel oral molecule KL1333 in healthy volunteers and subjects with primary mitochondrial disease. KL1333 aims to normalize the NAD+:NADH ratio that is critical for ATP production. The trial incorporated innovative design elements with potential translatability to other rare diseases including patient involvement, adaptive design and exploratory objectives, all of which have subsequently informed the protocol of an ongoing phase 2, pivotal efficacy study of KL1333. Results indicate KL1333 is safe and well tolerated, with dose-dependent gastrointestinal side effects, and validate potential novel outcome measures in primary mitochondrial disease including the 30-s Sit to Stand, and the patient-reported fatigue scales. Importantly, the data from the trial support efficacy of KL1333 based on improvements in fatigue and functional strength and endurance. Furthermore, the study highlights the value in using phase 1 studies to capture data that helps optimize later phase efficacy trial design.
Keywords: KL1333; clinical trial; phase 1 trial; primary mitochondrial disease; rare diseases.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
M.H., A.G., S.S.S., E.E. and M.J.H. are or have been employees and shareholders of Abliva AB who develops KL1333 for treatment of primary mitochondrial disease.
Figures

References
-
- Winter E, Schliebner S. Current advances in clinical trials for rare disease populations: Spotlight on the patient. Curr Rev Clin Exp Pharmacol. 2022;17:39–45. - PubMed
-
- Ng YS, Bindoff LA, Gorman GS, et al. . Mitochondrial disease in adults: Recent advances and future promise. Lancet Neurol. 2021;20:573–584. - PubMed
Publication types
MeSH terms
Grants and funding
- Muscular Dystrophy UK
- BRC
- Stoneygate Foundation
- Mitochondrial Research
- Tyne and Wear NHS Foundation Trust
- National Institute for Health Research Biomedical Research Centres
- MR/S005021/1/MitoCluster
- Rosetrees Trust
- Department of Health's
- Wellcome Centre
- LifeArc Centre to Treat Mitochondrial Diseases
- MRC_/Medical Research Council/United Kingdom
- University College London Hospitals
- UK NHS Highly Specialised Commissioners
- Newcastle University and Cumbria, Northumberland
- University College London
- Newcastle Hospitals NHS Foundation Trust
- The Lily Foundation
- MR/X02363X/1/Transition Support award
- Department of Health and Social Care.
- International Centre for Genomic Medicine in Neuromuscular Diseases
- a Medical Research Council
- 203105/WT_/Wellcome Trust/United Kingdom
- National Institute for Health and Care Research
- WT_/Wellcome Trust/United Kingdom
- Biomedical Research Centre
- National Mouse Genetics Network Mitochondria Cluster
- MR/S002065/1/Clinician Scientist Fellowship
- ICGNMD
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

