Discovery and adaptation of microbes that degrade oxidized low-density polyethylene films
- PMID: 39658361
- PMCID: PMC11664187
- DOI: 10.1093/jimb/kuae050
Discovery and adaptation of microbes that degrade oxidized low-density polyethylene films
Abstract
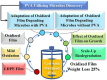
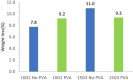
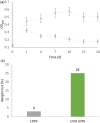
There is a growing interest in developing a methodology for effectively cleaving carbon-carbon (C-C) bonds in polymer backbones through bioconversion processes that utilize microorganisms and their enzymes. This upsurge of interest is driven by the goal of achieving a circular economy. Polyolefin post-consumer plastics are a substantial source of carbon, but the recycling potential is severely limited. Upcycling routes are needed for converting polyolefin post-consumer plastics into value-added products while concurrently mitigating adverse environmental effects. These materials contain carbon-based chemicals that can, in principle, serve as the feedstock for microbial metabolism. Some microbes have been reported to grow on polyolefin plastics, but the rate of biodegradation is insufficient for industrial processes. In this study, low-density polyethylene (LDPE) films were subjected to two mild ozone-based oxidation treatments, which facilitated biodegradation. The degree of oxidation was determined by Fourier transform infrared spectroscopy via analysis of the carbonyl index (1,710/1,460 cm-1), which ranged from 0.3 to 2.0, and also via analysis of the carboxylic acid content. Following oxidation of the films, studies were conducted to investigate the ability of a panel of polyvinyl alcohol-degrading microbes to degrade the oxidized films. A defined minimal medium was used to cultivate and assess microbial growth on the oxidized films. Following 45 days of cultivation, the most effective strains were further cultivated up to three additional generations on the oxidized film substrates to improve their ability to degrade the oxidized LDPE films. After these enrichments, we identified a strain from the third generation of Pseudomonas sp. Rh926 that exhibited significant cell growth and reduced the oxidized LDPE film mass by 25% in 30 days, demonstrating an enhanced capacity for degrading the oxidized LDPE films.
One-sentence summary: Discovery and adaptation techniques were used to enhance the metabolic capability of microorganisms for increased biodegradation of ozone-oxidized LDPE films as a step toward a future upcycling process.
Keywords: Adaptation; Biodegradation; Discovery; LDPE; Microbes; Oxidation.
© The Author(s) 2024. Published by Oxford University Press on behalf of Society of Industrial Microbiology and Biotechnology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Akhigbe G. E., EnochOghene A. E., Olumurewa K. O., Koleoso O., Ogbonna N. D. (2024). Characterization of low-density polyethylene (LDPE) films degraded using bacteria strains isolated from oil-contaminated soil. Environmental Technology, 45(16), 3155–3161. 10.1080/09593330.2023.2210770 - DOI - PubMed
-
- Albertson A. C., Karlsson S. (1990). The influence of biotic and biotic environments on the degradation of polyethylene. Progress in Polymer Science, 15(2), 177–192.
-
- Albertsson A. C., Andersson S. O., Karlsson S. (1987). The mechanism of biodegradation of polyethylene. Polymer Degradation and Stability, 18(1), 73–87.
-
- Badejo O., Hernández B., Vlachos D. G., Ierapetritou M. G. (2024). Design of sustainable supply chains for managing plastic waste: The case of low density polyethylene. Sustainable Production and Consumption, 47, 460–473. 10.1016/j.spc.2024.04.021 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

