The Core Circadian Clock Factor, Bmal1, Transduces Sex-specific Differences in Both Rhythmic and Nonrhythmic Gene Expression in the Mouse Heart
- PMID: 39658371
- PMCID: PMC11815582
- DOI: 10.1093/function/zqae053
The Core Circadian Clock Factor, Bmal1, Transduces Sex-specific Differences in Both Rhythmic and Nonrhythmic Gene Expression in the Mouse Heart
Abstract
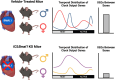
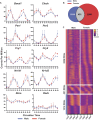
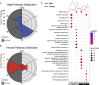
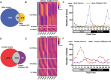
It has been well established that cardiovascular diseases exhibit significant differences between sexes in both preclinical models and humans. In addition, there is growing recognition that disrupted circadian rhythms can contribute to the onset and progression of cardiovascular diseases. However, little is known about sex differences between the cardiac circadian clock and circadian transcriptomes in mice. Here, we show that the core clock genes are expressed in common in both sexes, but the cardiac circadian transcriptome is very sex-specific. Hearts from female mice expressed significantly more rhythmically expressed genes (REGs) than male hearts, and the temporal distribution of REGs was distinctly different between sexes. To test the contribution of the circadian clock in sex-specific gene expression in the heart, we knocked out the core circadian clock factor Bmal1 in adult cardiomyocytes. The sex differences in the circadian transcriptomes were significantly diminished with cardiomyocyte-specific loss of Bmal1. Surprisingly, loss of cardiomyocyte Bmal1 also resulted in a roughly 8-fold reduction in the number of all differentially expressed genes between male and female hearts. We highlight sex-specific changes in several cardiac-specific transcription factors, including Gata4, Nkx2-5, and Tbx5. While there is still much to learn, we conclude that cardiomyocyte-specific Bmal1 is vital in conferring sex-specific gene expression in the adult mouse heart.
Keywords: brain and muscle ARNT-like 1; cardiomyocyte; circadian rhythms; sex differences.
© The Author(s) 2024. Published by Oxford University Press on behalf of American Physiological Society.
Conflict of interest statement
KAE holds the position of Editorial Board Member for Function and is blinded from reviewing or making decisions for the manuscript.
Figures



References
-
- Heron M. Deaths: leading causes for 2019. Natl Vital Stat Rep. 2021;70(9):1–114. - PubMed
-
- Regitz-Zagrosek V, Kararigas G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol Rev. 2017;97(1):1–37. - PubMed
-
- Cleland JGF, Swedberg K, Follath F et al. The EuroHeart Failure survey programme—a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J. 2003;24(5):442–463. - PubMed
-
- Douglas PS, Katz SE, Weinberg EO, Chen MH, Bishop SP, Lorell BH. Hypertrophic remodeling: gender differences in the early response to left ventricular pressure overload. J Am Coll Cardiol. 1998;32(4):1118–1125. - PubMed
-
- Crnko S, Du Pré BC, Sluijter JPG, Van Laake LW. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat Rev Cardiol. 2019;16(7):437–447. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
