Blood-based quantification of Aβ oligomers indicates impaired clearance from brain in ApoE ε4 positive subjects
- PMID: 39658587
- PMCID: PMC11631981
- DOI: 10.1038/s43856-024-00690-w
Blood-based quantification of Aβ oligomers indicates impaired clearance from brain in ApoE ε4 positive subjects
Abstract
Background: Quantification of Amyloid beta (Aβ) oligomers in plasma enables early diagnosis of Alzheimer's Disease (AD) and improves our understanding of underlying pathologies. However, quantification necessitates an extremely sensitive and selective technology because of very low Aβ oligomer concentrations and possible interference from matrix components.
Methods: In this report, we developed and validated a surface-based fluorescence distribution analysis (sFIDA) assay for quantification of Aβ oligomers in plasma.
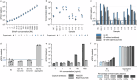
Results: The blood-based sFIDA assay delivers a sensitivity of 1.8 fM, an inter- and intra-assay variation below 20% for oligomer calibration standards and no interference with matrix components. Quantification of Aβ oligomers in 359 plasma samples from the DELCODE cohort reveals lower oligomer concentrations in subjective cognitive decline and AD patients than healthy Control participants.
Conclusions: Correlation analysis between CSF and plasma oligomer concentrations indicates an impaired clearance of Aβ oligomers that is dependent on the ApoE ε4 status.
Plain language summary
People with Alzheimer’s disease have difficulties with reasoning and communication. In Alzheimer’s disease, small proteins called amyloid beta (Aβ) stick together, forming tiny clusters in the brain that eventually grow larger. In this study, we aimed to measure these clusters in the blood. When we tested our method on blood samples from 359 people, we surprisingly found that people with Alzheimer’s disease and memory problems had fewer clusters of Aβ compared to healthy individuals. Our finding suggests that genetic factors may influence the body’s ability to clear these clusters from the brain.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: D.W. and O.B. are co-founders and shareholders of attyloid GmbH. This affiliation had no influence of the interpretation of the data. All other authors declare no competing interests related to this work.
Figures




References
-
- Mroczko, B., Groblewska, M., Litman-Zawadzka, A., Kornhuber, J. & Lewczuk, P. Amyloid beta oligomers (AbetaOs) in Alzheimer’s disease. J Neural Transm (Vienna)125, 177–191 (2018). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

