Electronic Sepsis Screening Among Patients Admitted to Hospital Wards: A Stepped-Wedge Cluster Randomized Trial
- PMID: 39658862
- PMCID: PMC11880955
- DOI: 10.1001/jama.2024.25982
Electronic Sepsis Screening Among Patients Admitted to Hospital Wards: A Stepped-Wedge Cluster Randomized Trial
Abstract
Importance: Sepsis screening is recommended among hospitalized patients but is supported by limited evidence of effectiveness.
Objective: To evaluate the effect of electronic sepsis screening, compared with no screening, on mortality among hospitalized ward patients.
Design, setting, and participants: In a stepped-wedge, cluster randomized trial at 5 hospitals in Saudi Arabia, 45 wards (clusters) were randomized into 9 sequences, 5 wards each, to have sepsis screening implemented at 2-month periods. The study was conducted between October 1, 2019, and July 31, 2021, with follow-up through October 29, 2021.
Intervention: An electronic alert, based on the quick Sequential Organ Failure Assessment score, was implemented in the electronic medical record in a silent mode that was activated to a revealed mode for sepsis screening.
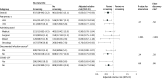
Main outcomes and measures: The primary outcome was 90-day in-hospital mortality. There were 11 secondary outcomes, including code blue activation, vasopressor therapy, incident kidney replacement therapy, multidrug-resistant organisms, and Clostridioides difficile.
Results: Among 60 055 patients, 29 442 were in the screening group and 30 613 in the no screening group. They had a median age of 59 years (IQR, 39-68), and 30 596 were male (51.0%). Alerts occurred in 4299 of 29 442 patients (14.6%) in the screening group and 5394 of 30 613 (17.6%) in the no screening group. Within 12 hours of the alert, patients in the screening group were more likely to have serum lactate tested (adjusted relative risk [aRR], 1.30; 95% CI, 1.16-1.45) and intravenous fluid ordered (aRR, 2.17; 95% CI, 1.92-2.46) compared with those in the no screening group. In the primary outcome analysis, electronic screening resulted in lower 90-day in-hospital mortality (aRR, 0.85; 95% CI, 0.77-0.93; P < .001). Screening reduced vasopressor therapy and multidrug-resistant organisms but increased code blue activation, incident kidney replacement therapy, and C difficile.
Conclusions and relevance: Among hospitalized ward patients, electronic sepsis screening compared with no screening resulted in significantly lower in-hospital 90-day mortality.
Trial registration: ClinicalTrials.gov Identifier: NCT04078594.
Conflict of interest statement
Figures

Comment in
-
Do Sepsis Alerts Help?JAMA. 2025 Mar 4;333(9):759-760. doi: 10.1001/jama.2024.25818. JAMA. 2025. PMID: 39658870 No abstract available.
References
-
- Dellinger RP, Levy MM, Rhodes A, et al. ; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup . Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165-228. doi: 10.1007/s00134-012-2769-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

